Numerical simulation and experimental verification on the diffusion behavior of tritium in zirconium alloy cladding materials
-
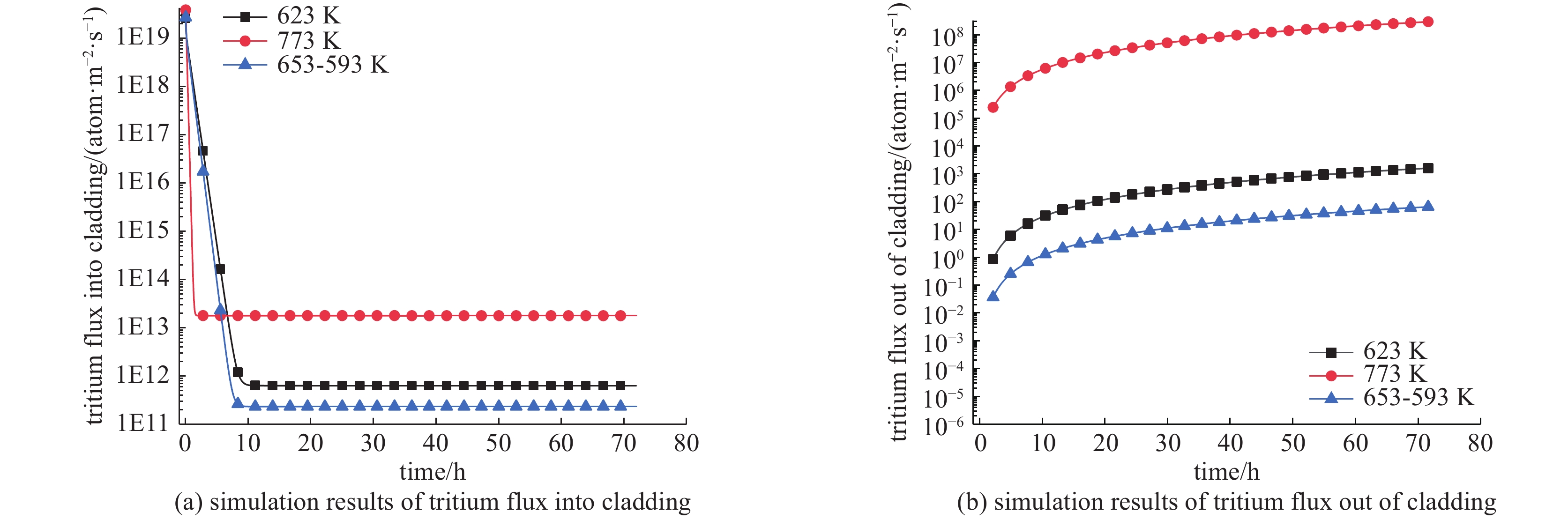
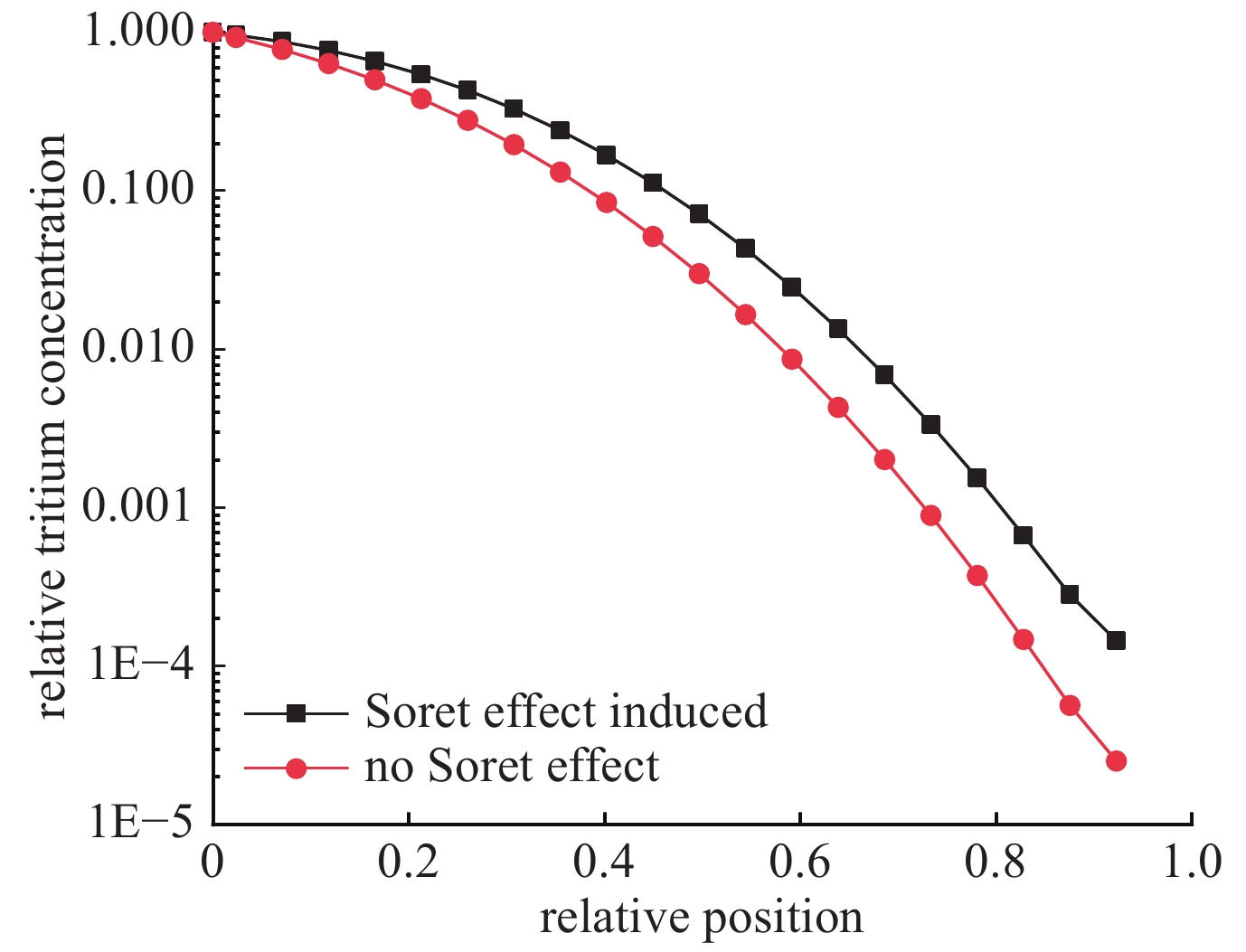
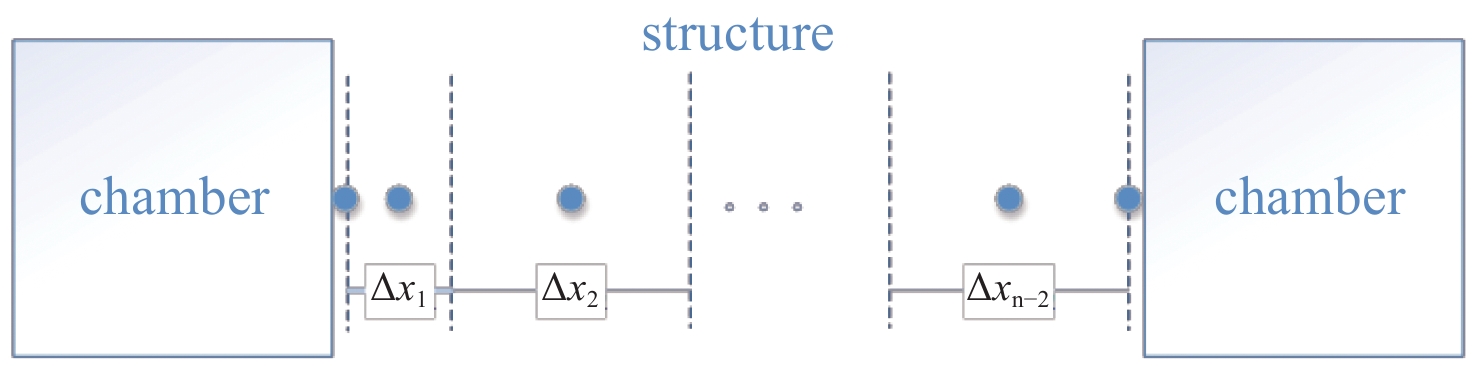
摘要: 基于氚扩散基本模型建立了氚扩散行为一维模拟程序,对模拟程序进行了典型实验验证,模拟结果与实验结果符合较好。分析了不同氚浓度、温度分布对锆合金包壳材料中氚的扩散行为的影响。分析结果表明:包壳-芯块间隙内氚浓度的升高会导致进出包壳的氚扩散通量提高,渗透通量增大;由于包壳氧化层相对较低的扩散系数,包壳氧化层的存在对氚渗透有较大的限制作用;温度对氚扩散速率的影响很显著,温度越高,扩散速度越快;锆合金外表面氧化层的相对低温限制了氚渗透出包壳管的速率,温度梯度导致的热致扩散有利于氚向包壳冷测扩散。Abstract: The research on the diffusion behavior of tritium in zirconium alloy is a crucial problem for nuclear power plant radiation safety evaluation. Based on the basic model of tritium diffusion, a one-dimensional simulation program for the behavior of tritium diffusion was established in this work. The simulation program was verified by typical experiments, and the simulation results were in good agreement with the experimental results. The effects of different concentration and temperature distribution of tritium on the diffusion behavior of tritium in zirconium alloy cladding materials were analyzed. The results show that the increase of tritium concentration in P-C gap lead to the increase of tritium diffusion flux and permeability flux in and out of cladding. Due to the relatively low diffusion coefficient of the coated oxide layer, the existence of the coated oxide layer limits the tritium permeability greatly. The effect of temperature on the diffusion rate of tritium is exponential. The higher the temperature is, the faster the diffusion rate is. The relative low temperature of the oxide layer on the outer surface of zirconium alloy limits the rate of tritium permeation out of the cladding tube. Thermally induced diffusion due to temperature gradient is beneficial to limit the permeation flux of tritium diffusing out of the zirconium cladding.
-
Key words:
- tritium /
- zirconium alloy /
- diffusion /
- numerical simulation
-
表 1 氘扩散实验参数表
Table 1. Parameters of the deuterium diffusion experiment
case thickness
(outer oxide layer)/μmthickness
(inner oxide layer)/μmsurface area/cm2 diffusion coefficient
(in outer oxide layer)/(cm2/s)diffusion coefficient
(in inner oxide layer)/(cm2/s)GNF-Ziron 0.5 1 4 2.2×10−13 1.6×10−14 VB 0.68 0.82 4 4.2×10−14 7.4×10−15 表 2 Surry-2燃料包壳氚释放实验参数
Table 2. Parameters of the tritium diffusion experiment of Surry-2 fuel caldding
parameter external diameter of
cladding/mminner diameter of
cladding/mmheight/mm surface area/m2 temperature/K burnup/
(GWd/MTU)thickness of dense
oxide layer/μmvalue 5.359 4.742 5.5 8.72664×10−5 773 36 2 -
[1] 陈海英, 张春明, 王韶伟. 压水堆3H源项计算分析[J]. 原子能科学技术, 2016, 50(2):459-463. (Chen Haiying, Zhang Chunming, Wang Shaowei. Calculation analysis of tritium source term in PWR[J]. Atomic Energy Science and Technology, 2016, 50(2): 459-463 [2] GB 6249-2011. 核动力厂环境辐射防护规定[S].GB 6249-2011. Regulations for environmental radiation protection of nuclear power plant[S] [3] Kearns J J. Diffusion coefficient of hydrogen in alpha zirconium, zircaloy-2, zircaloy-4[J]. Journal of Nuclear Materials, 1972, 43: 330-338. doi: 10.1016/0022-3115(72)90065-7 [4] Kunz W, Greger G U, Munzel H. Diffusion of tritium in zircaloy-2[J]. Journal of Nuclear Materials, 1980, 88: 15-22. doi: 10.1016/0022-3115(80)90381-5 [5] Khatamian D. Diffusion of hydrogen in single crystals of monoclinic-ZrO2 and yttrium stabilized cubic zirconia[J]. Defect and Diffusion Forum, 2010, 297/301: 631-640. doi: 10.4028/www.scientific.net/DDF.297-301.631 [6] Smith T. Kinetics and mechanism of hydrogen permeation of oxide films on zirconium[J]. Journal of Nuclear Materials, 1966, 18(3): 323-336. doi: 10.1016/0022-3115(66)90173-5 [7] Hanson D L, Richards M B, Connors G P.TRIGO code description and user’s guide[R], GA-20128-911081, 2006. [8] Eung S K, Chang H O, Patterson M. Study on the tritium behaviors in the VHTR system. Part 1: Development of tritium analysis code for VHTR and verification[J]. Nuclear Engineering and Design, 2010, 240: 1758-1767. doi: 10.1016/j.nucengdes.2010.02.023 [9] Longhurst G R. TMAP7 User Manual[R]. INL/EXT-04-02352, 2004. [10] Ohashi H, Sherman S. Tritium movement and accumulation in the NGNP system interface and hydrogen production[R]. INL/EXT-07-12746, 2007. [11] Yook D, Lee K J, Lee Y, et al. Estimation of the tritium behavior in the pebble type gas cooled reactor for hydrogen production[J]. Nuclear Science and Techniques, 2006, 43: 1522-1529. doi: 10.1080/18811248.2006.9711249 [12] Aly A, Avramova M, Ivanov K, et al. Three dimensional fuel pin model validation by prediction of hydrogen distribution in cladding and comparison with experiment[R]. DOE-NEUP-13-5180, 2017. [13] Yamanaka S, Miyake M, Katsura M. Hydrogen solubility in zirconium alloys[J]. Journal of Nuclear Materials, 1995, 247(1/2): 315-321. [14] Park M Y, Kim B Y, Kim E S. Development of semi-empirical model for tritium permeation under non-uniform temperature distribution at heat exchanger tube wall[J]. Annals of Nuclear Energy, 2015, 75: 413-420. doi: 10.1016/j.anucene.2014.08.044 [15] 黎辉, 梅其良, 付亚茹. 核电厂氚的产生和排放分析[J]. 原子能科学技术, 2015, 49(4):739-743. (Li Hui, Mei Qiliang, Fu Yaru. Analysis of generation and release of tritium in nuclear power plant[J]. Atomic Energy Science and Technology, 2015, 49(4): 739-743 doi: 10.7538/yzk.2015.49.04.0739 [16] Sawatzky A. The diffusion and solubility of hydrogen in the alpha-phase of zircaloy-2[J]. Journal of Nuclear Materials, 1960, 2(1): 62-68. doi: 10.1016/0022-3115(60)90025-8 [17] Takagi I, Une K, Miyamura S, et al. Deuterium diffusion in steam-corroded oxide layer of zirconium alloys[J]. Journal of Nuclear Material, 2011, 419: 339-346. doi: 10.1016/j.jnucmat.2011.06.001 [18] Robinson S M, Chattin M R, Giaquinto J M, et al. Evaluation of tritium content and release from pressurized water reactor fuel cladding[R]. ORNL/SPR-2015/425, 2015. [19] Barberis P, Frichet A. Characterization of zircaloy-4 oxide layers by impedance spectroscopy[J]. Journal of Nuclear Materials, 1999, 273: 182-191. doi: 10.1016/S0022-3115(99)00025-2 [20] Kammenzind B F, Franklin D G, Peters H R, et al. Hydrogen pickup and redistribution in alpha-annealed zircaloy-4[C]//11th International Symp on Zr in the Nuclear Industry. 1996: 338-370. -




 下载:
下载: