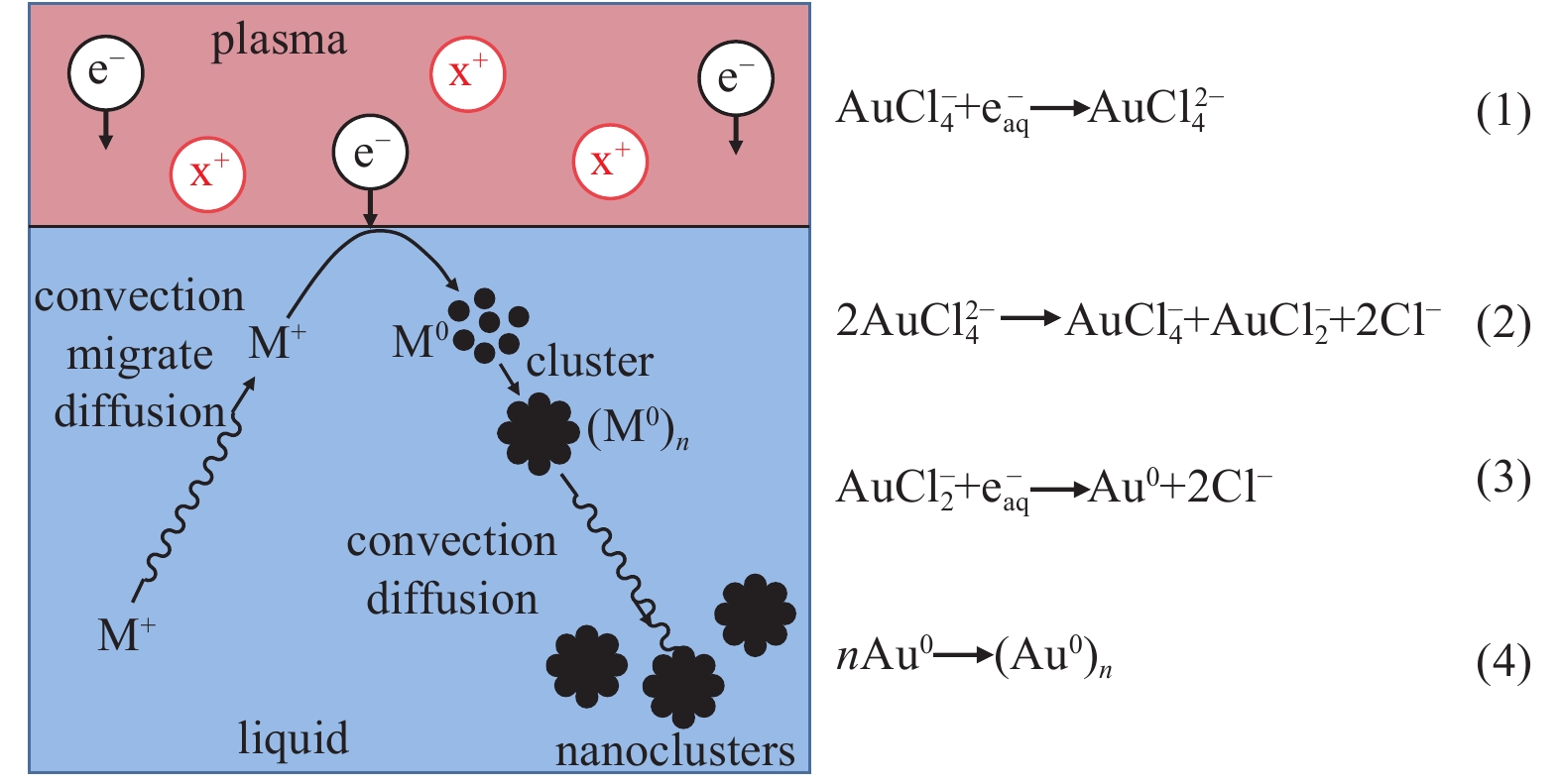
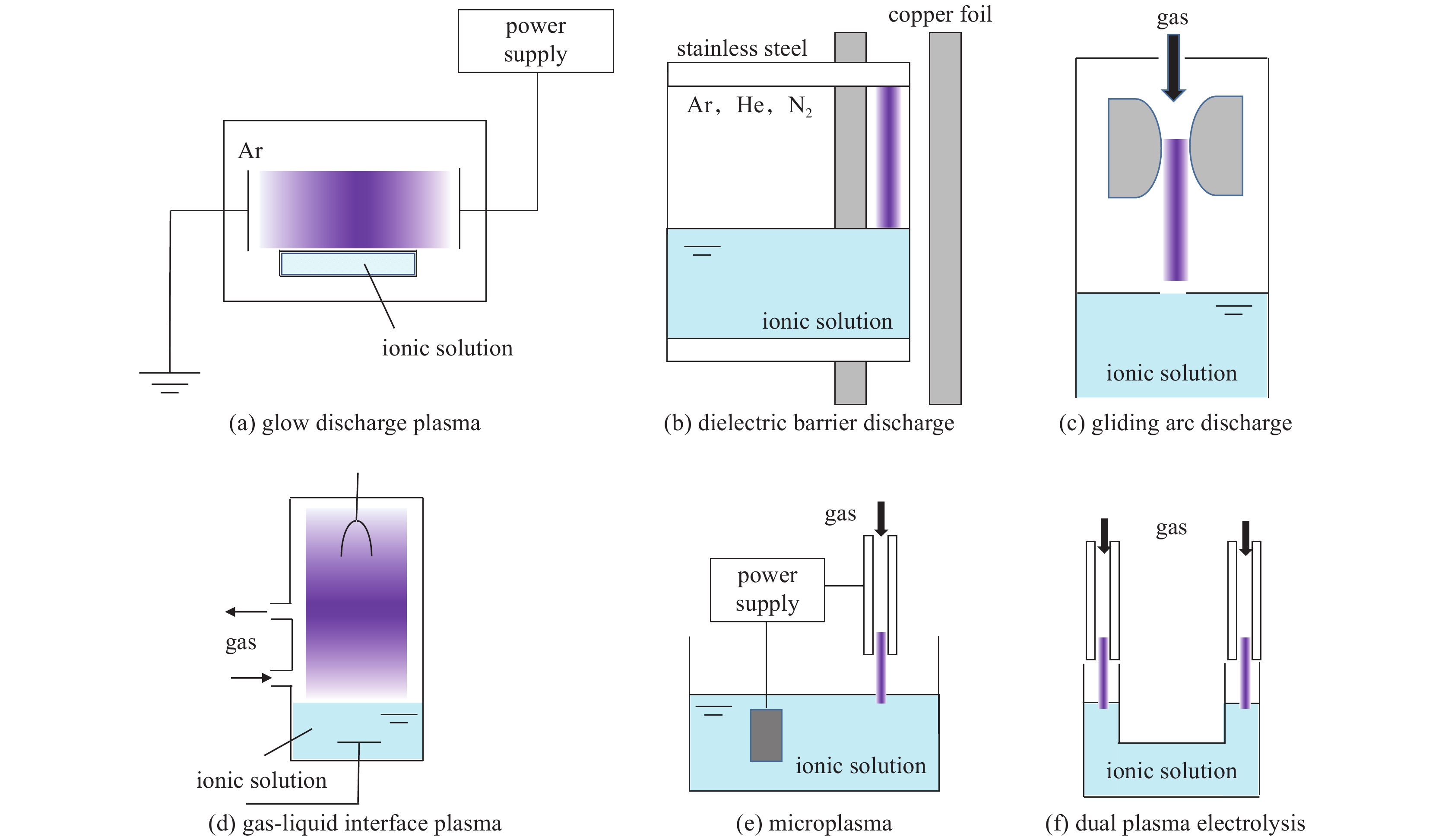
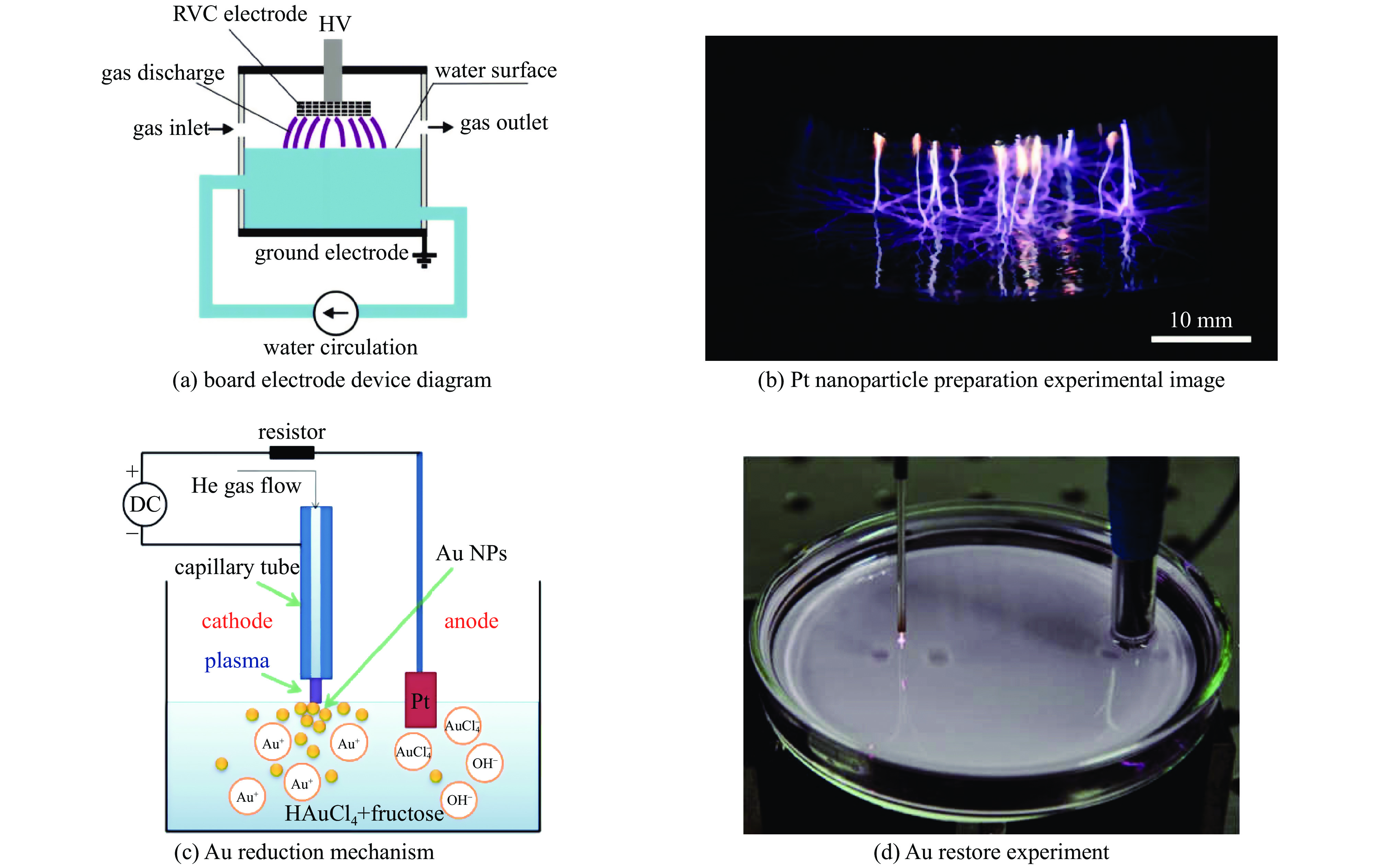
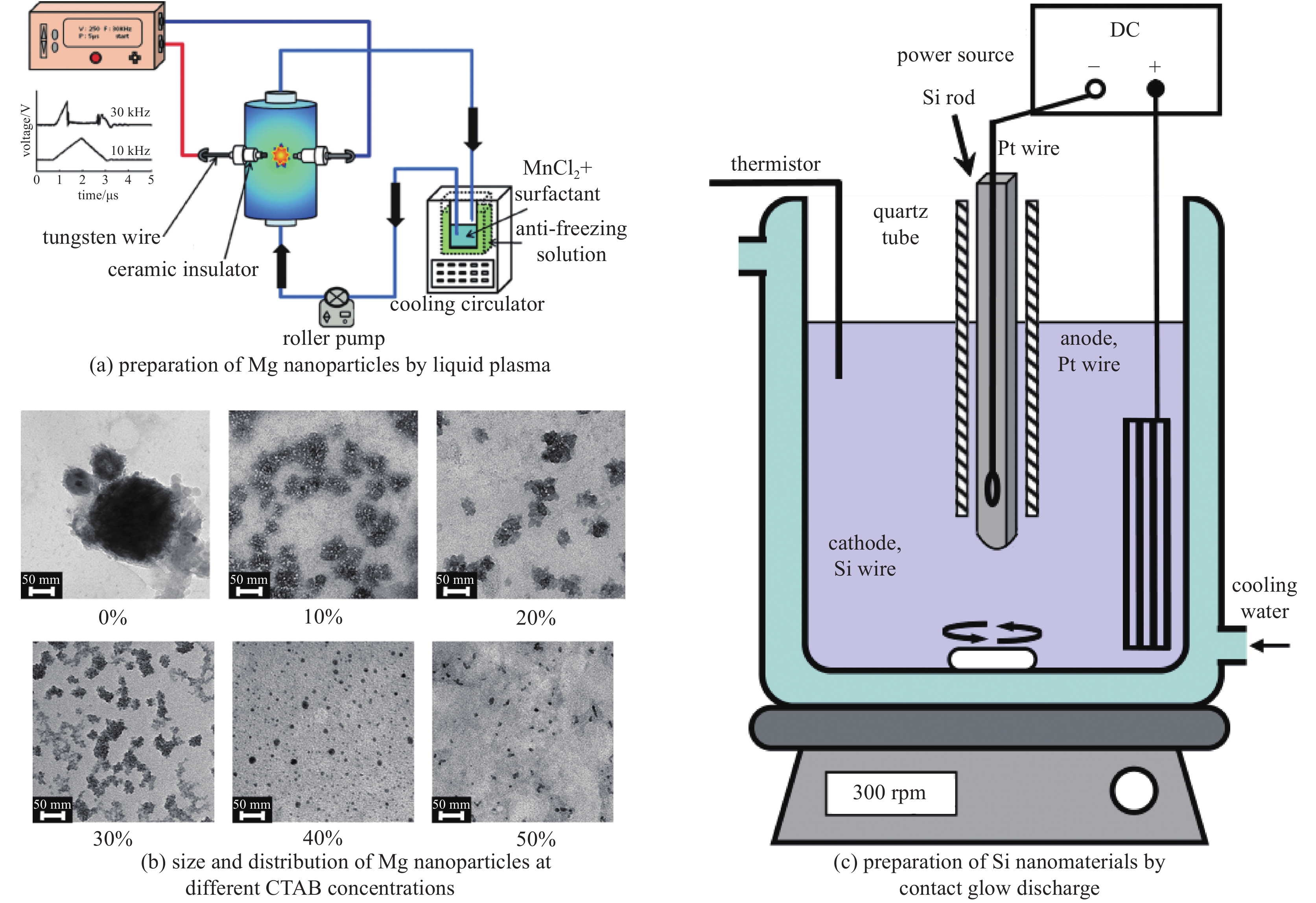
Liquid plasmas and their applications in nanomaterial synthesis
-
摘要:
液相等离子体是冷等离子体的一个新分支,具有温度低、传质传热快、常压操作、反应活性高等特点。基于液相等离子体的过程强化技术在纳米材料制备、挥发性有机物降解、杀菌消毒、化学合成等领域有广泛的应用前景。以液相等离子体中纳米材料的制备为研究对象,介绍了反应体系可能存在的活性粒子、检测方法和反应机理;对常见的反应器结构进行归纳整理,按照放电是否在电解液内部进行将其分为非浸没式和浸没式液相等离子体两大类,并列举了几种典型的反应器结构;介绍了几类利用液相等离子体技术制备纳米材料的典例,并对该领域的研究现状做了总结;对该领域亟需解决的问题与发展方向进行讨论与展望。
Abstract:As a new branch in non-thermal plasmas, plasma-liquid technology is characterized by low temperature, mass and heat transfer effectiveness, atmospheric-pressure operation and high reactivity. Process intensification techniques based on liquid plasmas have wide applications in nanofabrication, volatile organic compounds decomposition, sterilization and disinfection, chemical synthesis, etc. Taking the synthesis of nanomaterials by the liquid plasma as the research object, firstly, the reactive radicals that may exist in the system are introduced, together with the relevant characterization methods and possible reaction mechanisms; afterwards, the most widely adopted plasma-liquid systems are illustrated, which can be divided into non-immersed systems and immersed systems according to whether plasma is formed within the bulk of electrolytes; furthermore, several typical examples of nanomaterials synthesized by the plasma-liquid method are presented, with a summary of the state-of-art research in this field, finally, the challenges and development trends are discussed.
-
Key words:
- liquid plasmas /
- plasma equipment /
- nanotechnology /
- plasma-liquid interaction /
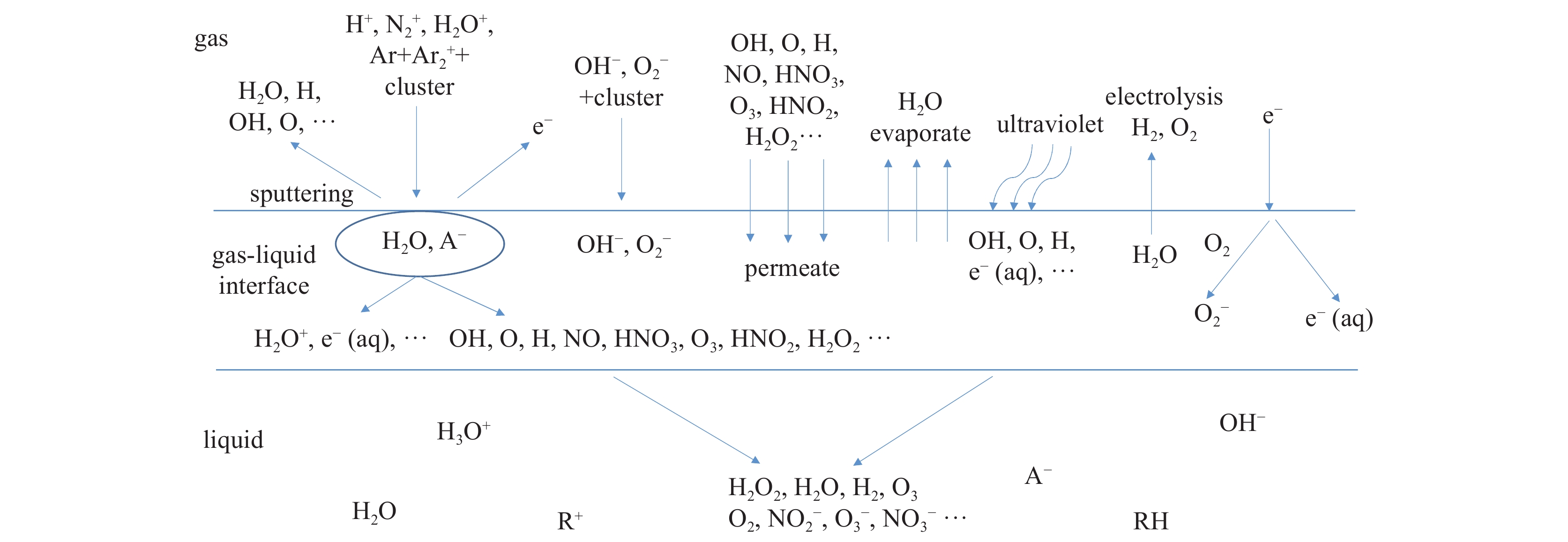
- nanomaterials
-
表 1 液相等离子体中常见自由基的检测方法及相关反应
Table 1. Detection methods and related reactions of common free radicals in liquid plasma
species detection method advantages and disadvantages correlation reaction references hydrogen peroxide
(H2O2)(1) titanium sulfate reagent colorimetry
(2) colorimetric or fluorometric analysis(1) less interference, good stability, but limited sensitivity(2) many interference factors (such as air, pH, solvent, etc.), but high sensitivity, lower detection limit~10×10−6 OH + H2O·→ H2O2+ H2O2+2H++2e−→H2O2H2O2+H++e−→H2O+OH [17][18][19] hydrogen
atom (H)(1) EPR technology
(2) isotope labeling(1) high sensitivity, low detection limit, easy decomposition of the product, requiring excess capture agent(2) the source, distribution and chemical reaction of H atoms can be explored, but it is usually used in conjunction with the EPR method H++e−→H [20][21][22] hydroxyl radicals
(OH·)(1) EPR technology(2) HPLC method
(3) spectrophotometry(1) high sensitivity and low detection limit, the most ideal method for the determination of hydroxyl radicals, the instrument is expensive, the choice of capture agent is critical(2) easy to implement, but its sensitivity and accuracy are still insufficient(3) easy to operate, has high selectivity, and is not easily affected by other ions at specific wavelengths; insufficient accuracy and sensitivity, requires the capture agent or the adduct product with the hydroxyl to have characteristic fluorescence H2O→H+OH·
OH·+H++e−→H2O[23][24][25][26] superoxide anion
(O2−)(1) EPR technology
(2) fluorescent probe method(1) high sensitivity, low detection limit, expensive instrument(2) easy to operate, lower instrument price, but the sensitivity is low, and it is interfered by particles like H2O2, OH· 2O+H2O+e−→H2O+O2− [27][28][29] ozone
(O3)(1) spectrophotometry
(2) fluorescent probe method(1) the operation is simple and fast, but the sensitivity is low, and it will be interfered by other oxidants(2) good selectivity, but it needs to be in a specific pH range; when pH ≥ 10, the probe will be destroyed in H2O2 O3+2H++2e−→O2+H2O [30][31][32] nitric oxide
(NO)(1) EPR technology(2) spectrophotometry
(3) fluorescent probe method(1) high sensitivity, low detection limit, wide application, but need to avoid oxidation of Fe2+ particles in the reagent(2) easy to operate, instrument is relatively cheap, and the aqueous solution to be tested needs to be acidified in advance before the test(3) has good selectivity, but the pH of the aqueous solution to be tested needs to be higher than 5.5 4NO+2O2+2H2O→
4NO2−+4H+[33][34][35][36] nitrite (NO2−) (1) spectrophotometry
(2) HPLC method(1) the operation is simple and fast, but the sensitivity is low, and it will be interfered by other oxidants(2) easy to implement, but its sensitivity and accuracy are still insufficient 2HNO2→NO+NO2+H2ONO2−+H+→HNO2 [37]
[38]nitrate
(NO3−)(1) spectrophotometry
(2) HPLC method(1) the operation is simple and fast, but the sensitivity is low, and it will be interfered by other oxidants(2) easy to implement, but its sensitivity and accuracy are still insufficient NO2−+ OH·→NO3− [39][40][41] peroxynitrite
(ONOO−)(1) colorimetric or fluorometric analysis(2) chemical reaction(3) fluorescent probe method (1) multiple interference factors, extremely sensitive to light-induced oxidation, and specificity problems(2) the existence of ONOO− can only be indirectly proved by the concentration decay of NO2− and H2O2, and the accuracy is insufficient (3) low selectivity, interfered by particles like H2O2 and HClO, in the absence ofHClO, the selectivity is enhanced NO2−+H2O2→ONOO−+H2OONOO−→NO3− [42][43][44] peroxynitric
acid (O2NOOH)(1) HPLC method (1) easy to detect, but the sensitivity is low, the detection environment requires low temperature and low pH value NO3−+H2O2→
O2NOO−+H2O[45] singlet oxygen
(1O2)(1) EPR technology
(2) fluorescent probe method(1) good selectivity, high sensitivity, not affected by other free radicals, the detection reagent has a strong pungent odor and is easily oxidized in the air(2) the probe acts as a photosensitizer to generate singlet oxygen, and the probe is destroyed in hydrogen peroxide O2+e−→1O2+e−21O2+2H+→H2O2+O21O2+H2O2→OH·+OH−+O2 [46][47][48][49] 表 2 基于液相等离子体技术合成纳米材料
Table 2. Synthesis of nanomaterials based on liquid phase plasma technology
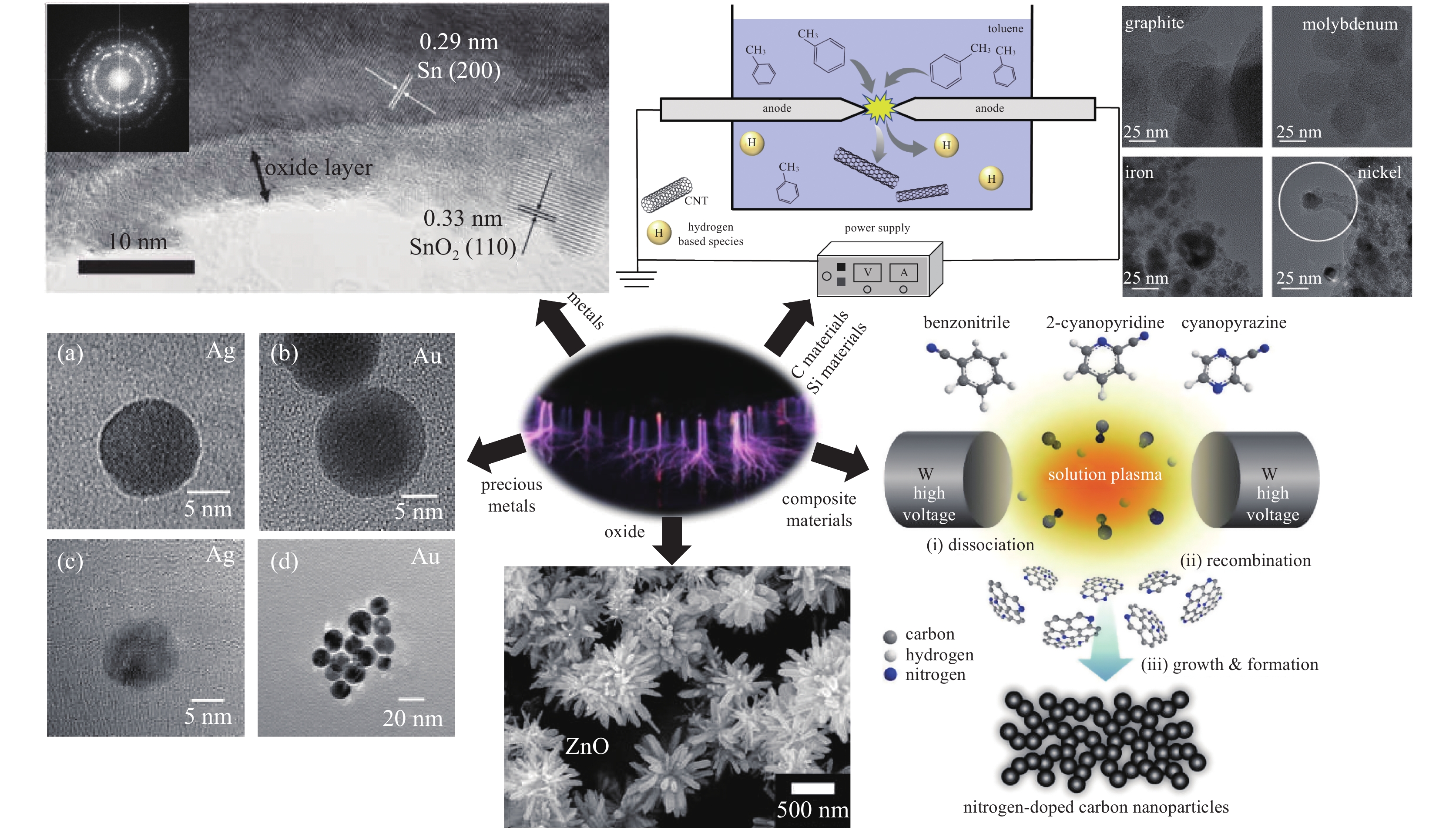
nanomaterials examples liquid plasma type references precious metal element Au contact glow discharge [56] microplasma discharge [63, 94] two-electrode pulsed plasma [76-81] Ag arc discharge [55, 84] Pd gas-liquid interface plasma [82] Pt flat electrode plasma [62] microplasma discharge [83] precious metal alloy Au - Ag alloy two-electrode plasma [54] microplasma discharge [60] Au - Pt alloy two-electrode pulsed plasma [85] metal element Ni solution glow discharge [57] Cu submerged arc discharge [86] Fe high voltage cathodic polarized discharge [58] Mn solution discharge [72] Sn dielectric barrier discharge [75] metal alloy Fe – Ni alloy solution discharge [59] Ni – Cu alloy dielectric barrier discharge [74] oxide CuO/ZnO two-electrode pulsed plasma [87] ZnO contact glow discharge [88] Cu2O microplasma discharge [95] TiO2 gliding arc discharge [52] defective TiO2 submerged pulse discharge [89] carbon material carbon nanotubes alternating current arc discharge [78] graphene submerged arc discharge [90-91] submerged arc discharge [79] silicon material Si dielectric barrier discharge [73] composite material carbon – metal nano contact glow discharge [92] carbon – non-metal nano solution discharge [93] plasma induced cathode discharge [96] -
[1] Ren Qiaoli, Ga Lu, Lu Zhili, et al. Aptamer-functionalized nanomaterials for biological applications[J]. Materials Chemistry Frontiers, 2020, 4(10): 1569-1585. [2] Shivashankar S A. Chemical synthesis of nanomaterials and structures, including nanostructured thin films, for different applications[M]//Vinoy K J, Ananthasuresh G K, Pratap R, et al. Micro and Smart Devices and Systems. New Delhi: Springer, 2014: 249-263. [3] Kulkarni S K. Synthesis of nanomaterials—I (physical methods)[M]//Kulkarni S K. Nanotechnology: Principles and Practices. Cham: Springer, 2015: 55-76. [4] Lin Liangliang, Starostin S A, Li Sirui, et al. Synthesis of metallic nanoparticles by microplasma[J]. Physical Sciences Reviews, 2018, 3(10): 20170121. [5] Lin Liangliang, Wang Qi. Microplasma: a new generation of technology for functional nanomaterial synthesis[J]. Plasma Chemistry and Plasma Processing, 2015, 35(6): 925-962. doi: 10.1007/s11090-015-9640-y [6] 冯雪兰, 程易. 气液等离子体过程强化技术及其在高级氧化过程的应用[J]. 化工进展, 2018, 37(4):1247-1256. (Feng Xuelan, Cheng Yi. Process intensification technique of gas-liquid plasma and its application in advanced oxidation processes[J]. Chemical Industry and Engineering Progress, 2018, 37(4): 1247-1256 [7] Tichonovas M, Krugly E, Racys V, et al. Degradation of various textile dyes as wastewater pollutants under dielectric barrier discharge plasma treatment[J]. Chemical Engineering Journal, 2013, 229: 9-19. doi: 10.1016/j.cej.2013.05.095 [8] 邓康, 胡小吐. 脉冲电弧液相放电等离子体污水消毒灭菌[J]. 环境工程学报, 2012, 6(10):3635-3638. (Deng Kang, Hu Xiaotu. Sterilization for wastewater by non-thermal plasma with pulsed arc electro-hydraulic discharge[J]. Chinese Journal of Environmental Engineering, 2012, 6(10): 3635-3638 [9] Toth J R, Abuyazid N H, Lacks D J, et al. A plasma-water droplet reactor for process-intensified, continuous nitrogen fixation at atmospheric pressure[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(39): 14845-14854. [10] Bruggeman P J, Kushner M J, Locke B R, et al. Plasma–liquid interactions: a review and roadmap[J]. Plasma Sources Science and Technology, 2016, 25: 053002. doi: 10.1088/0963-0252/25/5/053002 [11] 徐晗, 陈泽煜, 刘定新. 大气压冷等离子体处理水溶液: 液相活性粒子检测方法综述[J]. 电工技术学报, 2020, 35(17):3561-3582. (Xu Han, Chen Zeyu, Liu Dingxin. Aqueous solutions treated by cold atmospheric plasmas: a review of the detection methods of aqueous reactive species[J]. Transactions of China Electrotechnical Society, 2020, 35(17): 3561-3582 [12] Rabani J, Mulac W A, Matheson M S. The pulse radiolysis of aqueous tetranitromethane. I. Rate constants and the extinction coefficient of eaq−. II. Oxygenated solutions[J]. The Journal of Physical Chemistry, 1965, 69(1): 53-70. doi: 10.1021/j100885a011 [13] Hare P M, Price E A, Stanisky C M, et al. Solvated electron extinction coefficient and oscillator strength in high temperature water[J]. The Journal of Physical Chemistry A, 2010, 114(4): 1766-1775. doi: 10.1021/jp909789b [14] Hart E J, Anbar M. The hydrated electron[M]. New York, 1970. [15] Chen Qiang, Kaneko T, Hatakeyama R. Reductants in gold nanoparticle synthesis using gas–liquid interfacial discharge plasmas[J]. Applied Physics Express, 2012, 5: 086201. doi: 10.1143/APEX.5.086201 [16] Locke B R, Shih K Y. Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water[J]. Plasma Sources Science and Technology, 2011, 20: 034006. doi: 10.1088/0963-0252/20/3/034006 [17] Eisenberg G. Colorimetric determination of hydrogen peroxide[J]. Industrial and Engineering Chemistry, Analytical Edition, 1943, 15(5): 327-328. doi: 10.1021/i560117a011 [18] Winter J, Wende K, Masur K, et al. Feed gas humidity: a vital parameter affecting a cold atmospheric-pressure plasma jet and plasma-treated human skin cells[J]. Journal of Physics D:Applied Physics, 2013, 46: 295401. doi: 10.1088/0022-3727/46/29/295401 [19] Bratsch S G. Standard electrode potentials and temperature coefficients in water at 298.15 K[J]. Journal of Physical and Chemical Reference Data, 1989, 18(1): 1-21. doi: 10.1063/1.555839 [20] Gorbanev Y, O'Connell D, Chechik V. Non-thermal plasma in contact with water: the origin of species[J]. Chemistry - A European Journal, 2016, 22(10): 3496-3505. doi: 10.1002/chem.201503771 [21] Gorbanev Y, Verlackt C C W, Tinck S, et al. Combining experimental and modelling approaches to study the sources of reactive species induced in water by the COST RF plasma jet[J]. Physical Chemistry Chemical Physics, 2018, 20(4): 2797-2808. doi: 10.1039/C7CP07616A [22] Schwarz H A. Free radicals generated by radiolysis of aqueous solutions[J]. Journal of Chemical Education, 1981, 58: 101. doi: 10.1021/ed058p101 [23] Tresp H, Hammer M U, Winter J, et al. Quantitative detection of plasma-generated radicals in liquids by electron paramagnetic resonance spectroscopy[J]. Journal of Physics D:Applied Physics, 2013, 46: 435401. doi: 10.1088/0022-3727/46/43/435401 [24] Sahni M, Locke B R. Quantification of hydroxyl radicals produced in aqueous phase pulsed electrical discharge reactors[J]. Industrial & Engineering Chemistry Research, 2006, 45(17): 5819-5825. [25] Satoh A Y, Trosko J E, Masten S J. Methylene blue dye test for rapid qualitative detection of hydroxyl radicals formed in a Fenton’s reaction aqueous solution[J]. Environmental Science & Technology, 2007, 41(8): 2881-2887. [26] Chen Qiang, Li Junshuai, Li Yongfeng. A review of plasma–liquid interactions for nanomaterial synthesis[J]. Journal of Physics D: Applied Physics, 2015, 48: 424005. doi: 10.1088/0022-3727/48/42/424005 [27] Tani A, Fukui S, Ikawa S, et al. Diagnosis of superoxide anion radical induced in liquids by atmospheric-pressure plasma using superoxide dismutase[J]. Japanese Journal of Applied Physics, 2015, 54: 01AF01. doi: 10.7567/JJAP.54.01AF01 [28] Tang Bo, Zhang Li, Geng Yue. Determination of the antioxidant capacity of different food natural products with a new developed flow injection spectrofluorimetry detecting hydroxyl radicals[J]. Talanta, 2005, 65(3): 769-775. doi: 10.1016/j.talanta.2004.08.004 [29] Graham W G, Stalder K R. Plasmas in liquids and some of their applications in nanoscience[J]. Journal of Physics D: Applied Physics, 2011, 44: 174037. doi: 10.1088/0022-3727/44/17/174037 [30] Pavlovich M J, Chang H W, Sakiyama Y, et al. Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water[J]. Journal of Physics D: Applied Physics, 2013, 46: 145202. doi: 10.1088/0022-3727/46/14/145202 [31] GarneR A L, St Croix C M, Pitt B R, et al. Specific fluorogenic probes for ozone in biological and atmospheric samples[J]. Nature Chemistry, 2009, 1(4): 316-321. doi: 10.1038/nchem.240 [32] Bard A J, Faulkner L R. Electrochemical methods: fundamentals and applications[M]. New York: John Wiley & Sons, 1980: 410. [33] Zhang Qian, Sun Peng, Feng Hongqing, et al. Assessment of the roles of various inactivation agents in an argon-based direct current atmospheric pressure cold plasma jet[J]. Journal of Applied Physics, 2012, 111: 123305. doi: 10.1063/1.4730627 [34] Efrati S, Dishy V, Averbukh M, et al. The effect of N-acetylcysteine on renal function, nitric oxide, and oxidative stress after angiography[J]. Kidney International, 2003, 64(6): 2182-2187. doi: 10.1046/j.1523-1755.2003.00322.x [35] Shen Jie, Zhang Hao, Xu Zimu, et al. Preferential production of reactive species and bactericidal efficacy of gas-liquid plasma discharge[J]. Chemical Engineering Journal, 2019, 362: 402-412. doi: 10.1016/j.cej.2019.01.018 [36] Takamatsu T, Kawate A, Uehara K, et al. Bacterial inactivation in liquids using multi-gas plasmas[J]. Plasma Medicine, 2012, 2(4): 237-247. doi: 10.1615/PlasmaMed.2014010792 [37] 魏敬, 党文玲. 亚硝酸盐测定方法的比较与分析[J]. 肉类工业, 2004(7):37-38. (Wei Jing, Dang Wenling. Comparison and analysis of determination methods of nitrite[J]. Meat Industry, 2004(7): 37-38 doi: 10.3969/j.issn.1008-5467.2004.07.013 [38] Kieber R J, Seaton P J. Determination of subnanomolar concentrations of nitrite in natural waters[J]. Analytical Chemistry, 1995, 67(18): 3261-3264. doi: 10.1021/ac00114a024 [39] Machala Z, Tarabova B, Hensel K, et al. Formation of ROS and RNS in water electro-sprayed through transient spark discharge in air and their bactericidal effects[J]. Plasma Processes and Polymers, 2013, 10(7): 649-659. doi: 10.1002/ppap.201200113 [40] Oehmigen K, Hähnel M, Brandenburg R, et al. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids[J]. Plasma Processes and Polymers, 2010, 7(3/4): 250-257. [41] Chauvin J, Judée F, Yousfi M, et al. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet[J]. Scientific Reports, 2017, 7: 4562. doi: 10.1038/s41598-017-04650-4 [42] Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects[J]. Free Radical Biology and Medicine, 2007, 43(7): 995-1022. doi: 10.1016/j.freeradbiomed.2007.06.026 [43] Lukes P, Dolezalova E, Sisrova I, et al. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2[J]. Plasma Sources Science and Technology, 2014, 23: 015019. doi: 10.1088/0963-0252/23/1/015019 [44] Zielonka J, Zielonka M, Sikora A, et al. Global profiling of reactive oxygen and nitrogen species in biological systems[J]. Journal of Biological Chemistry, 2012, 287(5): 2984-2995. doi: 10.1074/jbc.M111.309062 [45] Nakashima Y, Ikawa S, Tani A, et al. Ion-exchange chromatographic analysis of peroxynitric acid[J]. Journal of Chromatography A, 2016, 1431: 89-93. doi: 10.1016/j.chroma.2015.12.054 [46] Davies M J. Singlet oxygen-mediated damage to proteins and its consequences[J]. Biochemical and Biophysical Research Communications, 2003, 305(3): 761-770. doi: 10.1016/S0006-291X(03)00817-9 [47] Ragàs X, Jiménez-Banzo A, Sánchez-García D, et al. Singlet oxygen photosensitisation by the fluorescent probe singlet oxygen sensor green[J]. Chemical Communications, 2009(20): 2920-2922. doi: 10.1039/b822776d [48] Arjunan K P, Clyne A M. Hydroxyl radical and hydrogen peroxide are primarily responsible for dielectric barrier discharge plasma-induced angiogenesis[J]. Plasma Processes and Polymers, 2011, 8(12): 1154-1164. doi: 10.1002/ppap.201100078 [49] Wu Haiyan, Sun Peng, Feng Hongqing, et al. Reactive oxygen species in a non-thermal plasma microjet and water system: generation, conversion, and contributions to bacteria inactivation—an analysis by electron spin resonance spectroscopy[J]. Plasma Processes and Polymers, 2012, 9(4): 417-424. doi: 10.1002/ppap.201100065 [50] Rumbach P, Bartels D M, Sankaran R M, et al. The solvation of electrons by an atmospheric-pressure plasma[J]. Nature Communications, 2015, 6: 7248. doi: 10.1038/ncomms8248 [51] Kitano K, Aoki H, Hamaguchi S. Radio-frequency-driven atmospheric-pressure plasmas in contact with liquid water[J]. Japanese Journal of Applied Physics, 2006, 45(10S): 8294-8297. [52] Acayanka E, Tiya Djowe A, Laminsi S, et al. Plasma-assisted synthesis of TiO2 nanorods by gliding arc discharge processing at atmospheric pressure for photocatalytic applications[J]. Plasma Chemistry and Plasma Processing, 2013, 33(4): 725-735. doi: 10.1007/s11090-013-9455-7 [53] Lin Liangliang, Ma Xintong, Li Sirui, et al. Plasma-electrochemical synthesis of europium doped cerium oxide nanoparticles[J]. Frontiers of Chemical Science and Engineering, 2019, 13(3): 501-510. doi: 10.1007/s11705-019-1810-7 [54] Shirai N, Uchida S, Tochikubo F. Synthesis of metal nanoparticles by dual plasma electrolysis using atmospheric dc glow discharge in contact with liquid[J]. Japanese Journal of Applied Physics, 2014, 53: 046202. doi: 10.7567/JJAP.53.046202 [55] Ashkarran A A, Iraji Zad A, Ahadian M M, et al. Stability, size and optical properties of colloidal silver nanoparticles prepared by electrical arc discharge in water[J]. The European Physical Journal Applied Physics, 2009, 48(1): 10601. doi: 10.1051/epjap/2009113 [56] Toriyabe Y, Watanabe S, Yatsu S, et al. Controlled formation of metallic nanoballs during plasma electrolysis[J]. Applied Physics Letters, 2007, 91: 041501. doi: 10.1063/1.2760042 [57] Saito G, Hosokai S, Akiyama T, et al. Size-controlled Ni nanoparticles formation by solution glow discharge[J]. Journal of the Physical Society of Japan, 2010, 79: 083501. doi: 10.1143/JPSJ.79.083501 [58] Tokushige M, Nishikiori T, Ito Y. Plasma-induced cathodic discharge electrolysis to form various metal/alloy nanoparticles[J]. Russian Journal of Electrochemistry, 2010, 46(6): 619-626. doi: 10.1134/S1023193510060042 [59] Lee W J, Park Y K, Kim J S, et al. Preparation and characterization of bimetallic Fe–Ni oxide nanoparticles using liquid phase plasma process[J]. Journal of Nanoscience and Nanotechnology, 2019, 19(4): 2362-2365. doi: 10.1166/jnn.2019.15988 [60] Yan Tingting, Zhong Xiaoxia, Rider A E, et al. Microplasma-chemical synthesis and tunable real-time plasmonic responses of alloyed AuxAg1−x nanoparticles[J]. Chemical Communications, 2014, 50(24): 3144-3147. doi: 10.1039/C3CC48846B [61] Saito G, Akiyama T. Nanomaterial synthesis using plasma generation in liquid[J]. Journal of Nanomaterials, 2015, 2015: 123696. [62] Kaneko T, Baba K, Harada T, et al. Novel gas-liquid interfacial plasmas for synthesis of metal nanoparticles[J]. Plasma Processes and Polymers, 2009, 6(11): 713-718. doi: 10.1002/ppap.200900029 [63] Huang Xunzhi, Li Yongsheng, Zhong Xiaoxia. Effect of experimental conditions on size control of Au nanoparticles synthesized by atmospheric microplasma electrochemistry[J]. Nanoscale Research Letters, 2014, 9: 572. doi: 10.1186/1556-276X-9-572 [64] Brenner M P, Hilgenfeldt S, Lohse D. Single-bubble sonoluminescence[J]. Reviews of Modern Physics, 2002, 74(2): 425-484. doi: 10.1103/RevModPhys.74.425 [65] De Giacomo A, Dell’Aglio M, De Pascale O, et al. From single pulse to double pulse ns-laser induced breakdown spectroscopy under water: elemental analysis of aqueous solutions and submerged solid samples[J]. Spectrochimica Acta Part B:Atomic Spectroscopy, 2007, 62(8): 721-738. doi: 10.1016/j.sab.2007.06.008 [66] Kim H J, Shin J G, Park C S, et al. In-liquid plasma process for size- and shape-controlled synthesis of silver nanoparticles by controlling gas bubbles in water[J]. Materials, 2018, 11: 891. doi: 10.3390/ma11060891 [67] Mashimo T, Tamura S, Yamamoto K, et al. Synthesis of Pd–Ru solid-solution nanoparticles by pulsed plasma in liquid method[J]. RSC Advances, 2020, 10(22): 13232-13236. doi: 10.1039/C9RA10003B [68] Ashkarran A A. A novel method for synthesis of colloidal silver nanoparticles by arc discharge in liquid[J]. Current Applied Physics, 2010, 10(6): 1442-1447. doi: 10.1016/j.cap.2010.05.010 [69] Saito G, Azman W O S B W M, Nakasugi Y, et al. Optimization of electrolyte concentration and voltage for effective formation of Sn/SnO2 nanoparticles by electrolysis in liquid[J]. Advanced Powder Technology, 2014, 25(3): 1038-1042. doi: 10.1016/j.apt.2014.02.003 [70] Azumi K, Mizuno T, Akimoto T, et al. Light emission from Pt during high-voltage cathodic polarization[J]. Journal of the Electrochemical Society, 1999, 146(9): 3374-3377. doi: 10.1149/1.1392480 [71] Mizuno T, Akimoto T, Azumi K, et al. Hydrogen evolution by plasma electrolysis in aqueous solution[J]. Japanese Journal of Applied Physics, 2005, 44: 396. doi: 10.1143/JJAP.44.396 [72] Kim H G, Lee H, Kim S J, et al. Synthesis of manganese nanoparticles in the liquid phase plasma[J]. Journal of Nanoscience and Nanotechnology, 2013, 13(9): 6103-6108. doi: 10.1166/jnn.2013.7681 [73] Saito G, Sakaguchi N. Solution plasma synthesis of Si nanoparticles[J]. Nanotechnology, 2015, 26: 235602. doi: 10.1088/0957-4484/26/23/235602 [74] Saito G, Nakasugi Y, Yamashita T, et al. Solution plasma synthesis of bimetallic nanoparticles[J]. Nanotechnology, 2014, 25: 135603. doi: 10.1088/0957-4484/25/13/135603 [75] Saito G, Zhu Chunyu, Akiyama T. Surfactant-assisted synthesis of Sn nanoparticles via solution plasma technique[J]. Advanced Powder Technology, 2014, 25(2): 728-732. doi: 10.1016/j.apt.2013.11.001 [76] Hu Xiulan, Takai O, Saito N. Synthesis of gold nanoparticles by solution plasma sputtering in various solvents[J]. Journal of Physics: Conference Series, 2013, 417: 012030. doi: 10.1088/1742-6596/417/1/012030 [77] Hu Xiulan, Cho S P, Takai O, et al. Rapid synthesis and structural characterization of well-defined gold clusters by solution plasma sputtering[J]. Crystal Growth & Design, 2012, 12(1): 119-123. [78] Biró L P, Horváth Z E, Szalmás L, et al. Continuous carbon nanotube production in underwater AC electric arc[J]. Chemical Physics Letters, 2015, 372(3/4): 399-402. [79] Scuderi V, Bongiorno C, Faraci G, et al. Effect of the liquid environment on the formation of carbon nanotubes and graphene layers by arcing processes[J]. Carbon, 2012, 50(6): 2365-2369. doi: 10.1016/j.carbon.2012.01.053 [80] 邱瑞. 微波等离子体化学气相沉积系统研究[D]. 成都: 电子科技大学, 2012Qiu Rui. Research on microwave plasma chemical vapor deposition system[D]. Chengdu: University of Electronic Science and Technology of China, 2012 [81] Treesukkasem N, Chokradjaroen C, Theeramunkong S, et al. Synthesis of Au nanoparticles in natural matrices by liquid-phase plasma: effects on cytotoxic activity against normal and cancer cell lines[J]. ACS Applied Nano Materials, 2019, 2(12): 8051-8062. doi: 10.1021/acsanm.9b02106 [82] Höfft O, Endres F. Plasma electrochemistry in ionic liquids: an alternative route to generate nanoparticles[J]. Physical Chemistry Chemical Physics, 2011, 13(30): 13472-13478. doi: 10.1039/c1cp20501c [83] Xu Hujun, He Chaohong, Lin Liangliang, et al. Direct formation of carbon supported Pt nanoparticles by plasma-based technique[J]. Materials Letters, 2019, 255: 126532. doi: 10.1016/j.matlet.2019.126532 [84] Richmonds C, Sankaran R M. Plasma-liquid electrochemistry: rapid synthesis of colloidal metal nanoparticles by microplasma reduction of aqueous cations[J]. Applied Physics Letters, 2008, 93: 131501. doi: 10.1063/1.2988283 [85] Hu Xiulan, Shen Xiaodong, Takai O, et al. Facile fabrication of PtAu alloy clusters using solution plasma sputtering and their electrocatalytic activity[J]. Journal of Alloys and Compounds, 2013, 552: 351-355. doi: 10.1016/j.jallcom.2012.08.033 [86] Xie Suyuan, Ma Zhijie, Wang Chunfang, et al. Preparation and self-assembly of copper nanoparticles via discharge of copper rod electrodes in a surfactant solution: a combination of physical and chemical processes[J]. Journal of Solid State Chemistry, 2004, 177(10): 3743-3747. doi: 10.1016/j.jssc.2004.07.012 [87] Hu Xiulan, Zhang Xin, Shen Xiaodong, et al. Plasma-induced synthesis of CuO nanofibers and ZnO nanoflowers in water[J]. Plasma Chemistry and Plasma Processing, 2014, 34(5): 1129-1139. doi: 10.1007/s11090-014-9546-0 [88] Saito G, Hosokai S, Akiyama T. Synthesis of ZnO nanoflowers by solution plasma[J]. Materials Chemistry and Physics, 2011, 130(1/2): 79-83. [89] Jedsukontorn T, Ueno T, Saito N, et al. Facile preparation of defective black TiO2 through the solution plasma process: effect of parametric changes for plasma discharge on its structural and optical properties[J]. Journal of Alloys and Compounds, 2017, 726: 567-577. doi: 10.1016/j.jallcom.2017.08.028 [90] Sano N, Nakano J, Kanki T. Synthesis of single-walled carbon nanotubes with nanohorns by arc in liquid nitrogen[J]. Carbon, 2004, 42(3): 686-688. doi: 10.1016/j.carbon.2003.12.078 [91] Okada T, Kaneko T, Hatakeyama R. Conversion of toluene into carbon nanotubes using arc discharge plasmas in solution[J]. Thin Solid Films, 2007, 515(9): 4262-4265. doi: 10.1016/j.tsf.2006.02.067 [92] Hamdan A, Kabbara H, Courty M A, et al. Synthesis of carbon–metal multi-strand nanocomposites by discharges in heptane between two metallic electrodes[J]. Plasma Chemistry and Plasma Processing, 2017, 37(4): 1069-1090. doi: 10.1007/s11090-017-9816-8 [93] Panomsuwan G, Saito N, Ishizaki T. Electrocatalytic oxygen reduction on nitrogen-doped carbon nanoparticles derived from cyano-aromatic molecules via a solution plasma approach[J]. Carbon, 2016, 98: 411-420. doi: 10.1016/j.carbon.2015.11.013 [94] Li Xuanhe, Lin Liangliang, Chiang W H, et al. Microplasma synthesized gold nanoparticles for surface enhanced Raman spectroscopic detection of methylene blue[J]. Reaction Chemistry & Engineering, 2022, 7(2): 346-353. [95] Liu Jiandi, He Bangbang, Chen Qiang, et al. Plasma electrochemical synthesis of cuprous oxide nanoparticles and their visible-light photocatalytic effect[J]. Electrochimica Acta, 2016, 222: 1677-1681. doi: 10.1016/j.electacta.2016.11.158 [96] Tokushige M, Tsujimura H, Nishikiori T, et al. Formation of metallic Si and SiC nanoparticles from SiO2 particles by plasma-induced cathodic discharge electrolysis in chloride melt[J]. Electrochimica Acta, 2013, 100: 300-303. doi: 10.1016/j.electacta.2012.08.068 -




 下载:
下载: