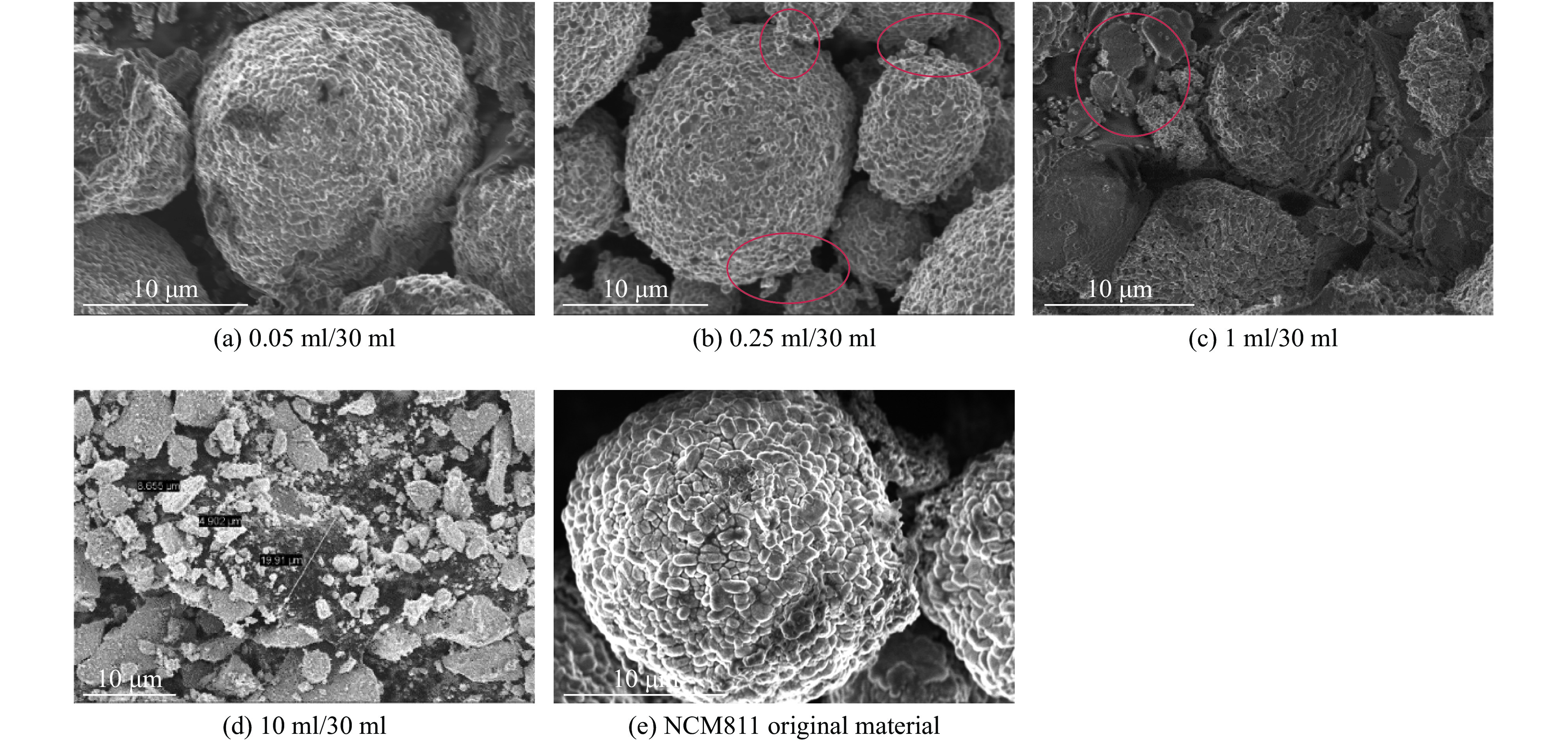
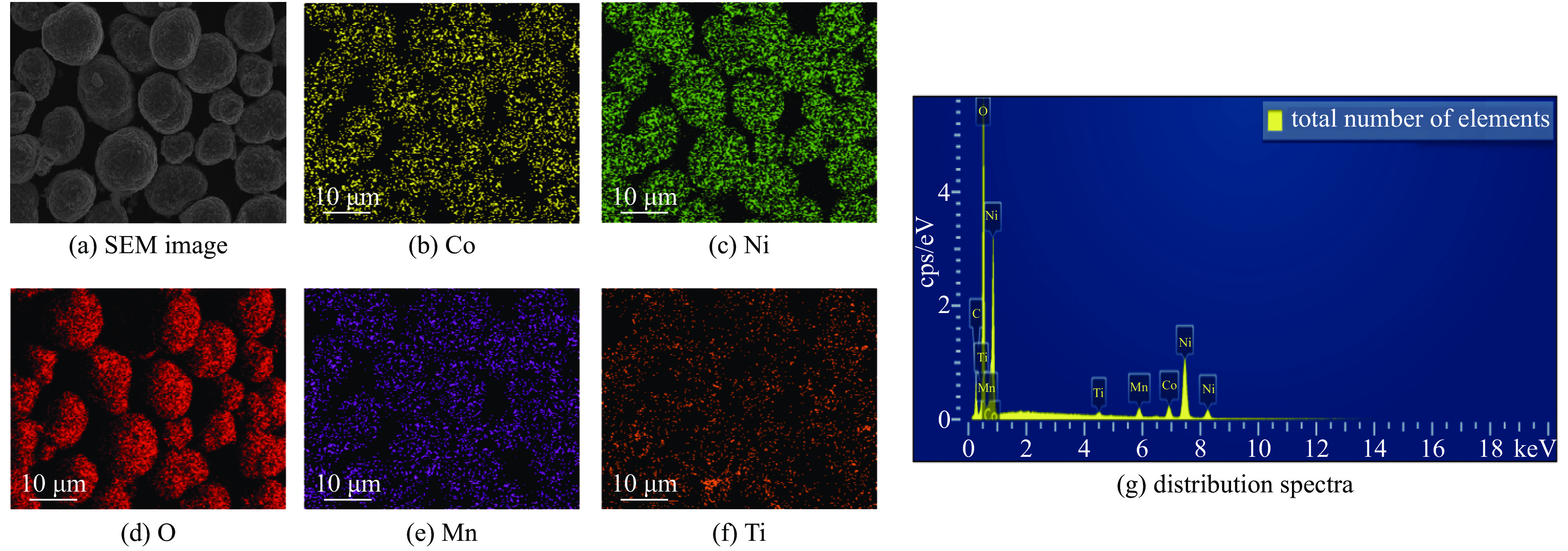
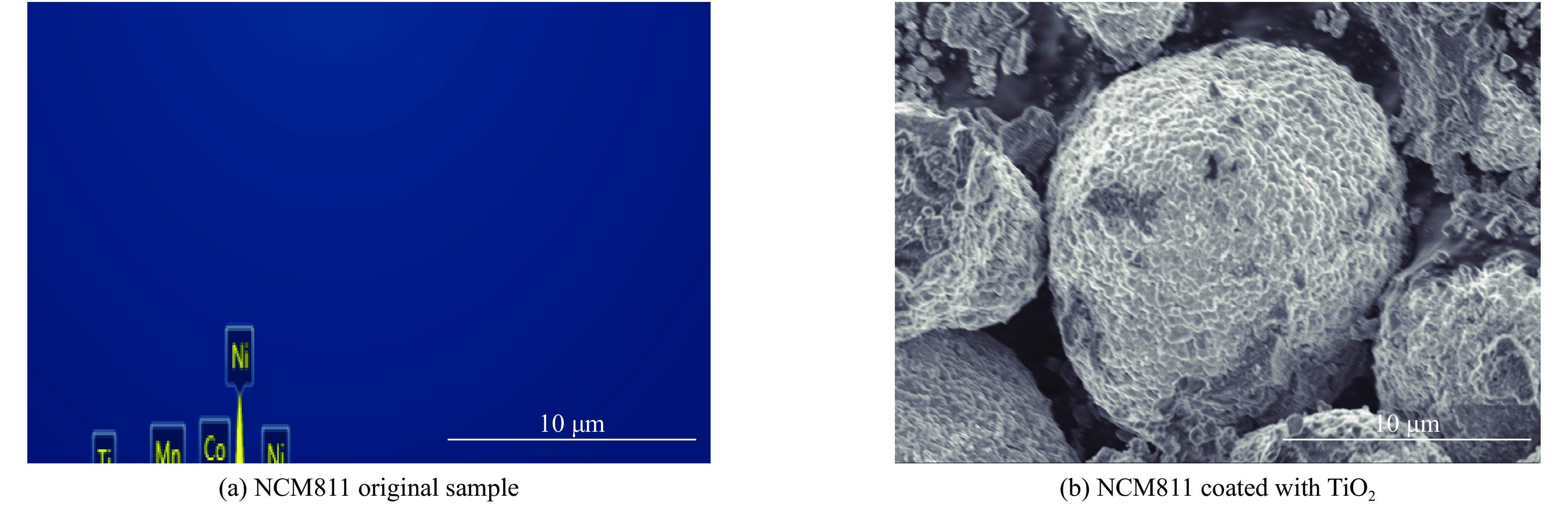
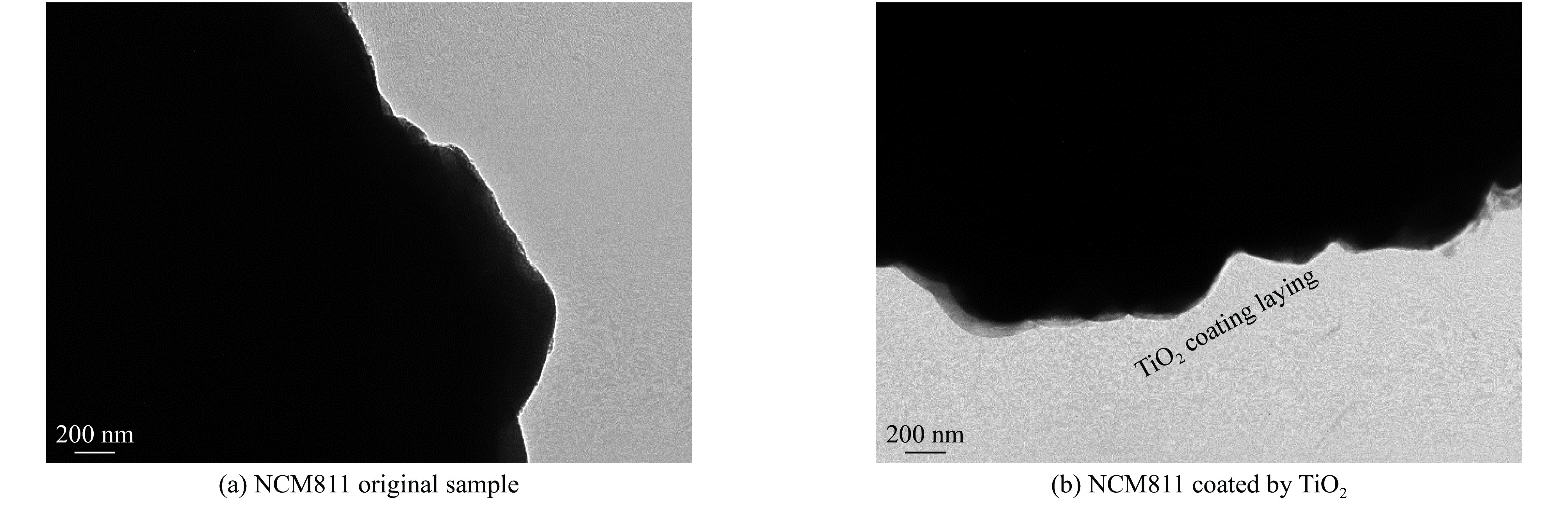
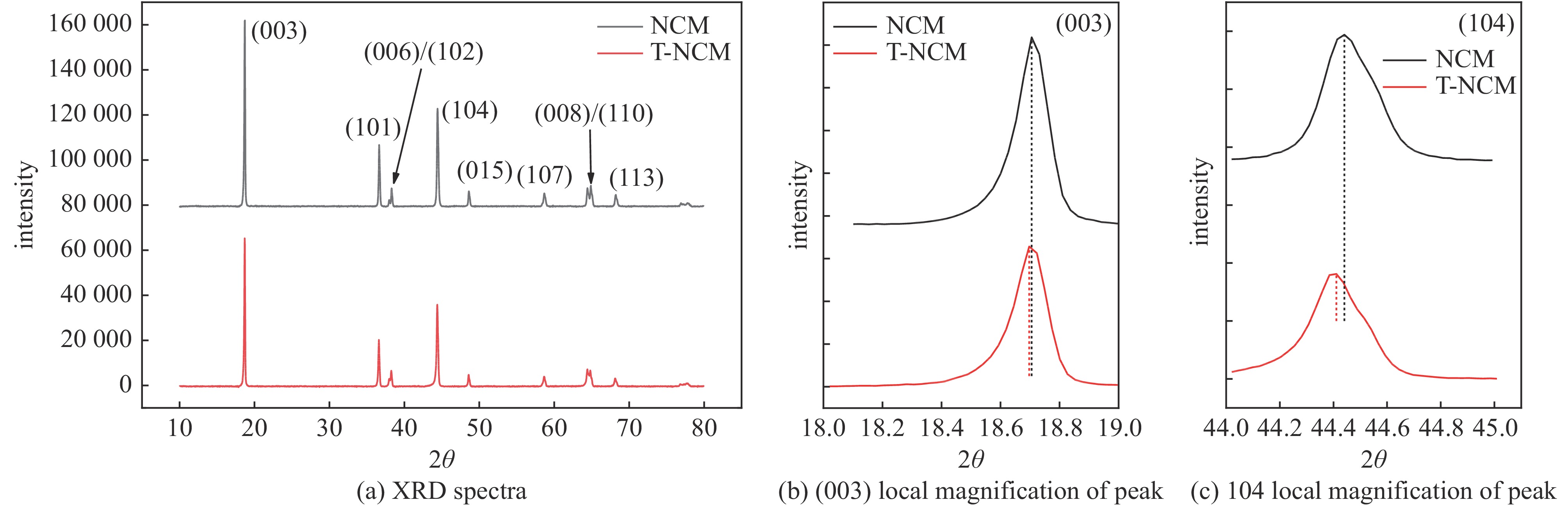
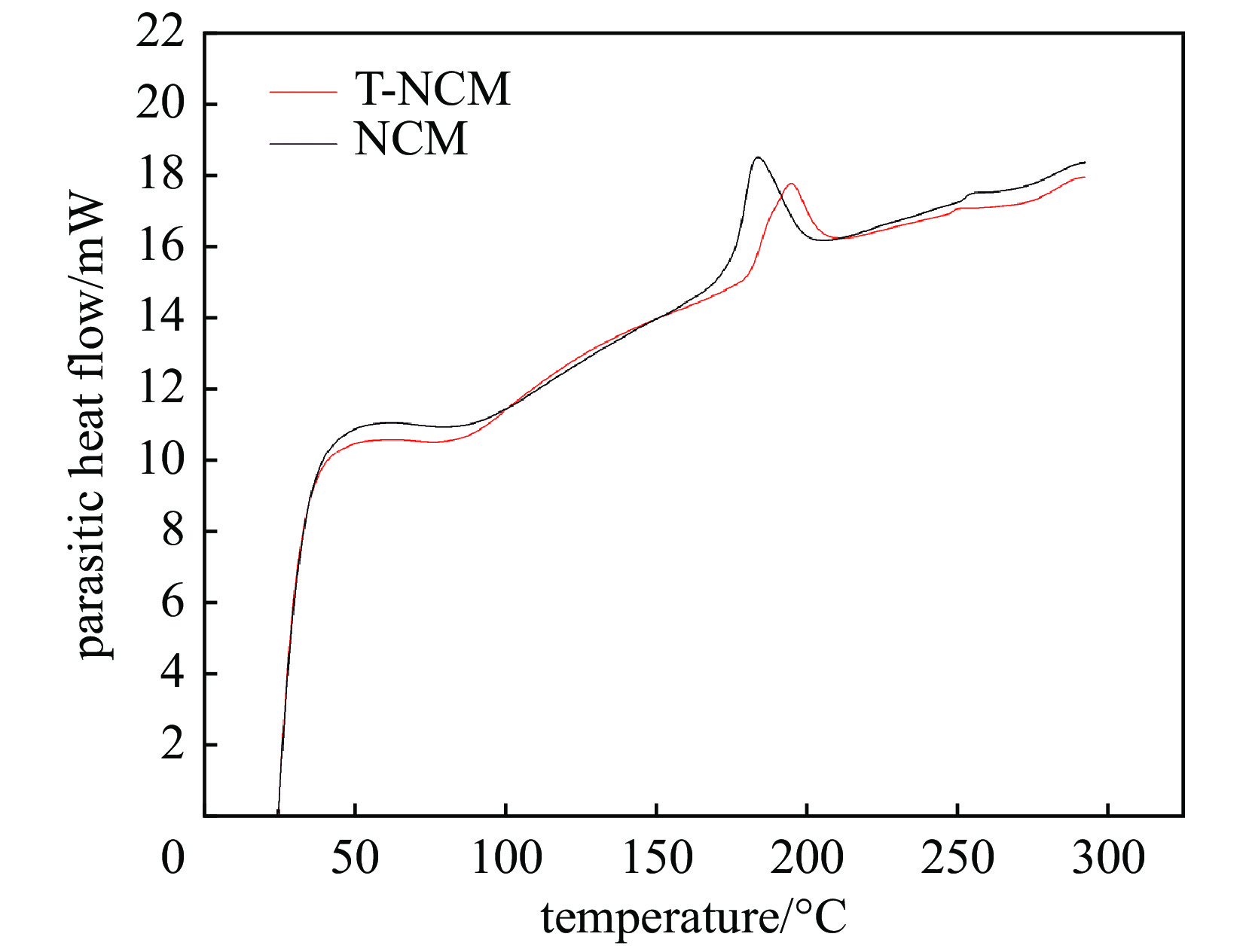
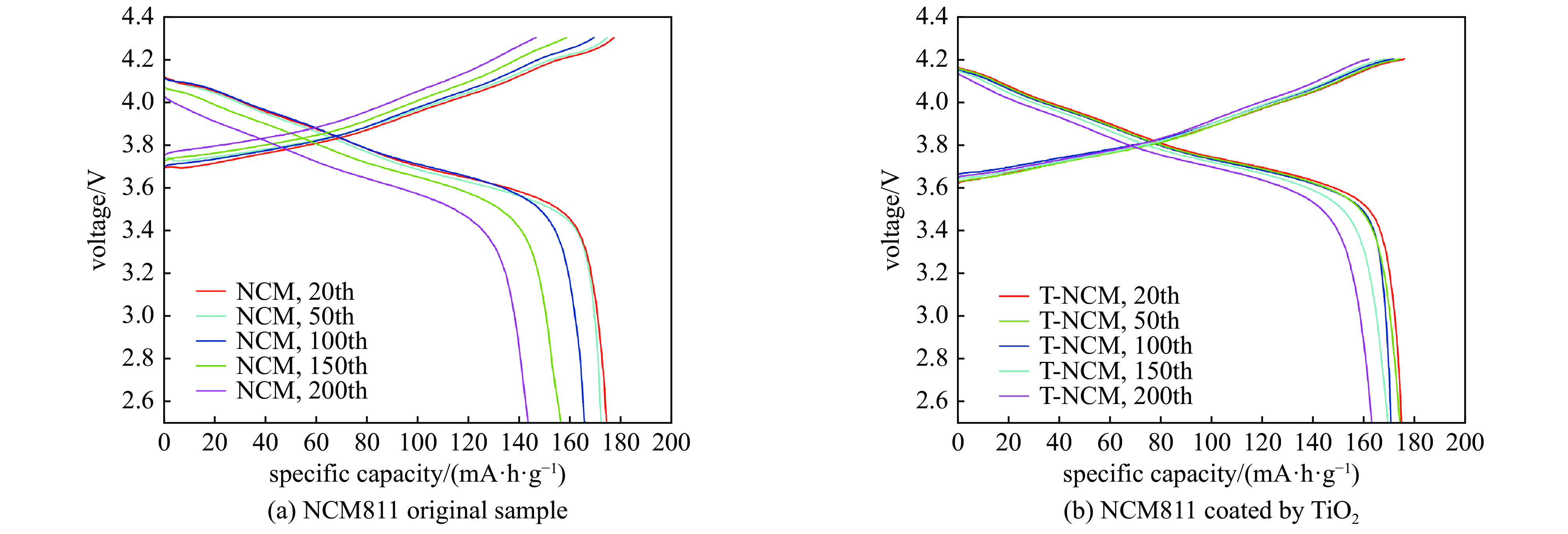
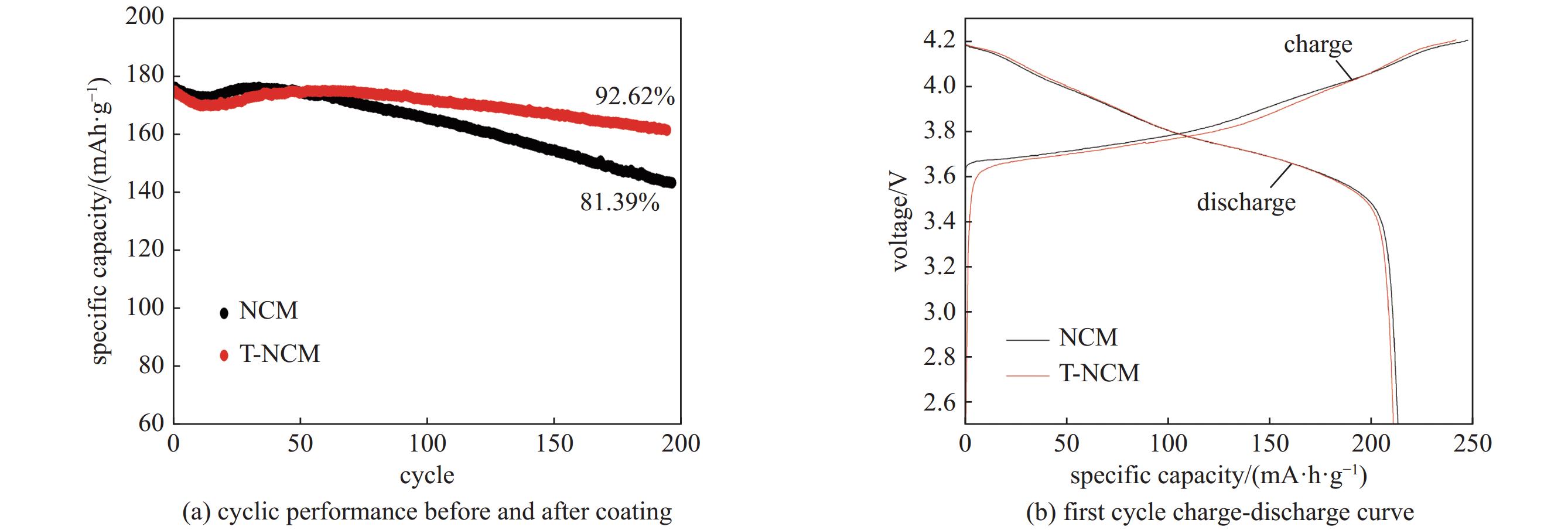
| [1] |
Thackeray M M, Amine K. Layered Li–Ni–Mn–Co oxide cathodes[J]. Nature Energy, 2021, 6(9): 933. doi: 10.1038/s41560-021-00860-3
|
| [2] |
Yin Shouyi, Deng Wentao, Chen Jun, et al. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries[J]. Nano Energy, 2021, 83: 105854. doi: 10.1016/j.nanoen.2021.105854
|
| [3] |
Yin L, Li Z, Mattei G S. Thermodynamics of antisite defects in layered NMC cathodes: systematic insights from high-precision powder diffraction analyses[J]. Chemistry of Materials, 2020, 32(3): 1002-1010. doi: 10.1021/acs.chemmater.9b03646
|
| [4] |
Gwon H, Kim S W, Park Y U, et al. Ion-exchange mechanism of layered transition-metal oxides: case study of LiNi0.5Mn0.5O2[J]. Inorganic Chemistry, 2014, 53(15): 8083-8087. doi: 10.1021/ic501069x
|
| [5] |
Meng Kui, Wang Zhixing, Guo Huajun, et al. Enhanced cycling stability of LiNi0.8Co0.1Mn0.1O2 by reducing surface oxygen defects[J]. Electrochimica Acta, 2017, 234: 99-107. doi: 10.1016/j.electacta.2017.03.054
|
| [6] |
Xiao Yinguo, Liu Tongchao, Liu Jiajie, et al. Insight into the origin of lithium/nickel ions exchange in layered Li(Ni x Mn y Co z )O2 cathode materials[J]. Nano Energy, 2018, 49: 77-85. doi: 10.1016/j.nanoen.2018.04.020
|
| [7] |
Hu Qiao, He Yufang, Ren Dongsheng, et al. Targeted masking enables stable cycling of LiNi0.6Co0.2Mn0.2O2 at 4.6V[J]. Nano Energy, 2022, 96: 107123. doi: 10.1016/j.nanoen.2022.107123
|
| [8] |
Yoon M, Dong Yanhao, Hwang J. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries[J]. Nature Energy, 2021, 6(4): 362-371. doi: 10.1038/s41560-021-00782-0
|
| [9] |
Hall D S, Gauthier R, Eldesoky A, et al. New chemical insights into the beneficial role of Al2O3 cathode coatings in lithium-ion cells[J]. ACS Applied Materials & Interfaces, 2019, 11(15): 14095-14100.
|
| [10] |
Fan Qinglu, Lin Kaiji, Yang Shaodian, et al. Constructing effective TiO2 nano-coating for high-voltage Ni-rich cathode materials for lithium ion batteries by precise kinetic control[J]. Journal of Power Sources, 2020, 477: 228745. doi: 10.1016/j.jpowsour.2020.228745
|
| [11] |
Ma Yuan, Teo J H, Walther F, et al. Advanced nanoparticle coatings for stabilizing layered Ni-rich oxide cathodes in solid-state batteries[J]. Advanced Functional Materials, 2022, 32: 2111829. doi: 10.1002/adfm.202111829
|
| [12] |
Hua Weibo, Zhang Jibin, Zheng Zhuo, et al. Na-doped Ni-rich LiNi0.5Co0.2Mn0.3O2 cathode material with both high rate capability and high tap density for lithium ion batteries[J]. Dalton Transactions, 2014, 43(39): 14824-14832. doi: 10.1039/C4DT01611D
|
| [13] |
Yang Zuguang, Guo Xiaodong, Xiang Wei, et al. K-doped layered LiNi0.5Co0.2Mn0.3O2 cathode material: Towards the superior rate capability and cycling performance[J]. Journal of Alloys and Compounds, 2017, 699: 358-365. doi: 10.1016/j.jallcom.2016.11.245
|
| [14] |
Kim Y, Park H, Shin K, et al. Rational design of coating ions via advantageous surface reconstruction in high-nickel layered oxide cathodes for lithium-ion batteries[J]. Advanced Energy Materials, 2021, 11(38): 2101112. doi: 10.1002/aenm.202101112
|




 下载:
下载: