Preparation and electrochemical performances of ternary LiNi1-x-yCoxMnyO2 cathode material
-
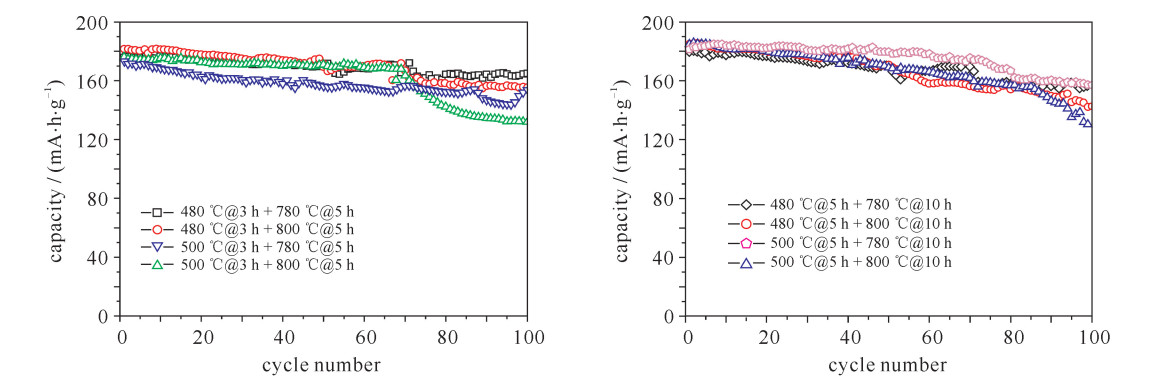
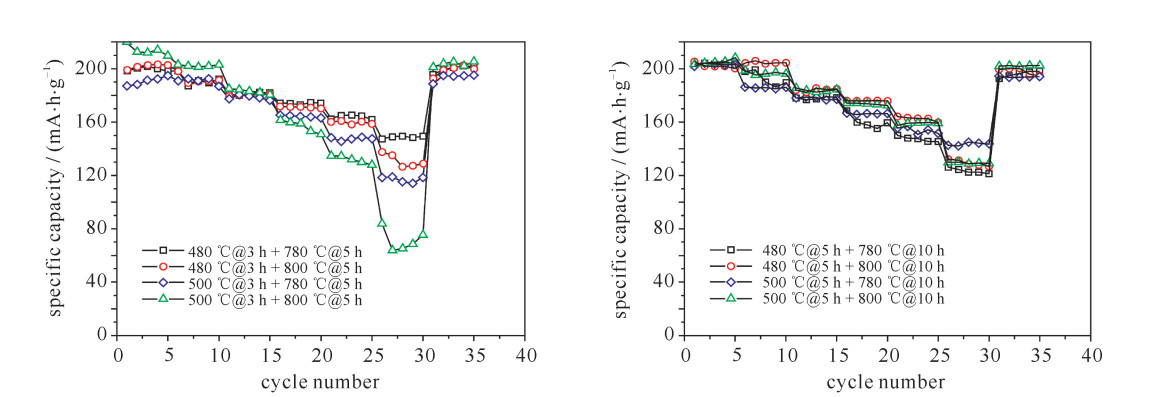
摘要: LiNi1-x-yCoxMnyO2正极材料作为最有商业化前途的锂离子电池正极材料,近年来成为研究者关注的焦点。但目前针对该材料合成工艺的研究还较少。对LiNi0.8Co0.15Mn0.05O2开展了不同的烧结工艺研究,并对制备出的正极材料进行了表征和性能测试。研究发现在0.1C (电池容量额定值)倍率下充放电比容量为200 mA·h·g-1左右,在1C倍率循环100次下,480 ℃@3 h + 780 ℃@5 h和500 ℃@5 h + 780 ℃@10 h两种烧结工艺下容量保持率分别为94%和86%,说明用这两种工艺制备的正极材料的综合性能最优。Abstract: As the most promising commercial lithium-ion battery, the LiNi1-x-yCoxMnyO2 cathode have become the focus of researchers in recent years. However, there are less researches on the synthetic process of this material. This paper discusses various sintering processes of LiNi0.8Co0.15Mn0.05O2, and the characterization and electrochemical performances testing of the prepared cathode materials. The research shows that the material has higher specific capacity (about 200 mA·h·g-1) at 0.1C (C is battery capacity rating value) charging-discharging rate. At 1C and 100 times of charging-discharging, the capacity retention ratios of 480 ℃@3 h + 780 ℃@5 h and 500 ℃@5 h + 780 ℃@10 h sintering processes are 94% and 86%, respectively, which illustrates the synthetical properties of the cathode materials prepared by these two processes are optimal.
-
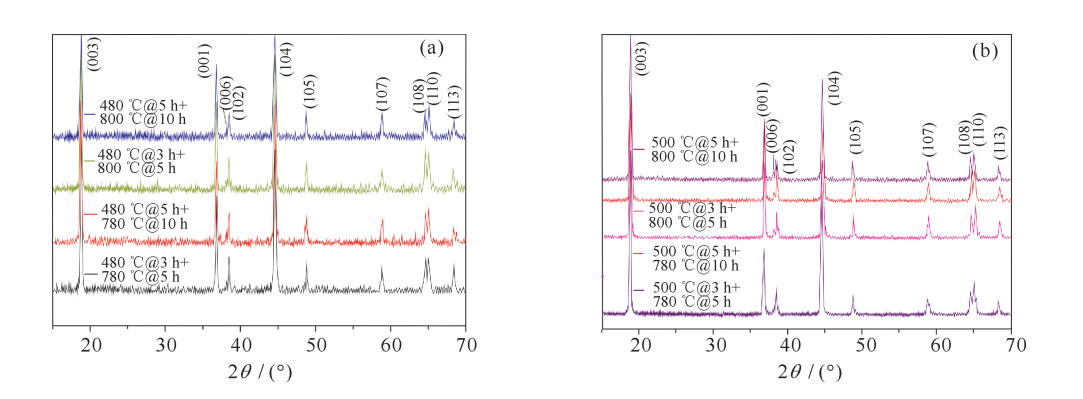
表 1 正极材料LiNi0.8Co0.15Mn0.05O2的烧结工艺
Table 1. Sintering process of cathode material LiNi0.8Co0.15Mn0.05O2
No presintering temperature/℃ presintering time/h calcination temperature/℃ calcination time/h remarks 1 20→480(120 min) 3 480→780 (60 min) 5 cooling 2 20→480(120 min) 5 480→780(60 min) 10 cooling 3 20→480(120 min) 3 480→800(64 min) 5 cooling 4 20→480(120 min) 5 480→800(64 min) 10 cooling 5 20→500(124 min) 3 500→780(56 min) 5 cooling 6 20→500(124 min) 5 500→780(56 min) 10 cooling 7 20→500(124 min) 3 500→800(60 min) 5 cooling 8 20→500(124 min) 5 500→800(60 min) 10 cooling 表 2 不同烧结工艺下正极材料的NCM晶格参数
Table 2. NCM lattice parameters of cathode material under different sintering processes
sample a/nm c/nm c/a I(003) I(104) R=I(003)/I(104) 480 ℃@3 h+780 ℃@5 h 0.250 74 1.409 47 5.621 2 100 54.2 1.845 0 480 ℃@3 h+800 ℃@5 h 0.250 48 1.410 10 5.629 6 100 51.0 1.960 8 500℃@3 h+780 ℃@5 h 0.286 45 1.410 94 4.925 6 100 57.4 1.742 2 500℃@3 h+800 ℃@5 h 0.286 38 1.407 10 4.913 4 100 58.9 1.697 8 480 ℃@5 h+780 ℃@10 h 0.250 39 1.410 24 5.632 2 100 54.9 1.821 5 480 ℃@5 h+800 ℃@10 h 0.250 56 1.409 18 5.624 1 100 63.1 1.584 8 500℃@5 h+780 ℃@10 h 0.286 20 1.407 21 4.916 9 100 47.6 2.100 8 500℃@5 h+800 ℃@10 h 0.286 37 1.411 11 4.927 6 100 57.5 1.739 1 表 3 不同烧结工艺下正极材料EIS拟合结果
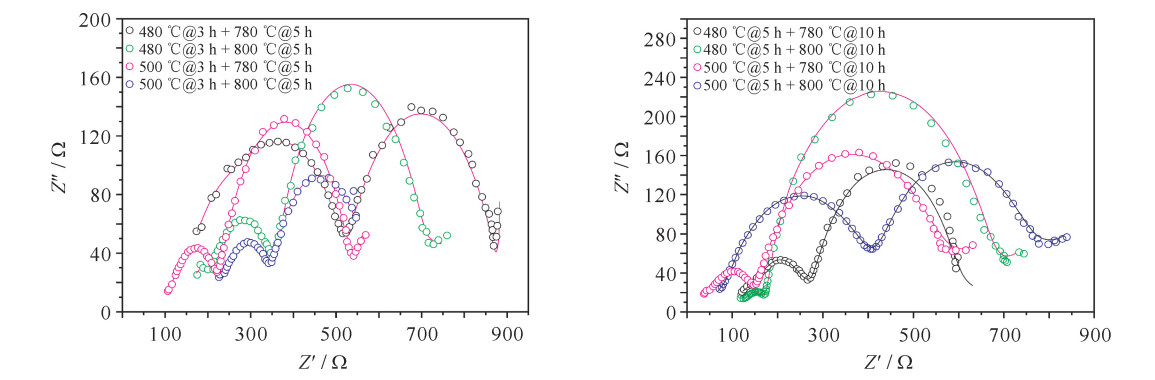
Table 3. EIS fitting results of cathode material under different sintering processes
sample electrolyte resistivity/Ω RSEI/Ω resistency/Ω Rct/Ω 480 ℃@3 h+780 ℃@5 h 135.40 171.70 211.50 366.20 480 ℃@3 h+800 ℃@5 h 153.50 310.60 146.60 44.84 500℃@3 h+780 ℃@5 h 90.93 43.19 100.30 293.60 500℃@3 h+800 ℃@5 h 0.001 253 237.40 218.50 111.10 480 ℃@5 h+780 ℃@10 h 3.933 271.00 86.11 20.65 480 ℃@5 h+800 ℃@10 h 0.544 1 31.76 134.50 460.50 500℃@5 h+780 ℃@10 h 17.00 36.86 360.60 80.89 500℃@5 h+800 ℃@10 h 27.21 312.7 47.51 276.70 表 4 各材料CV曲线对应的主要氧化还原峰电位及电位差
Table 4. Main REDOX peak potential and PD corresponding to CV curve of each material
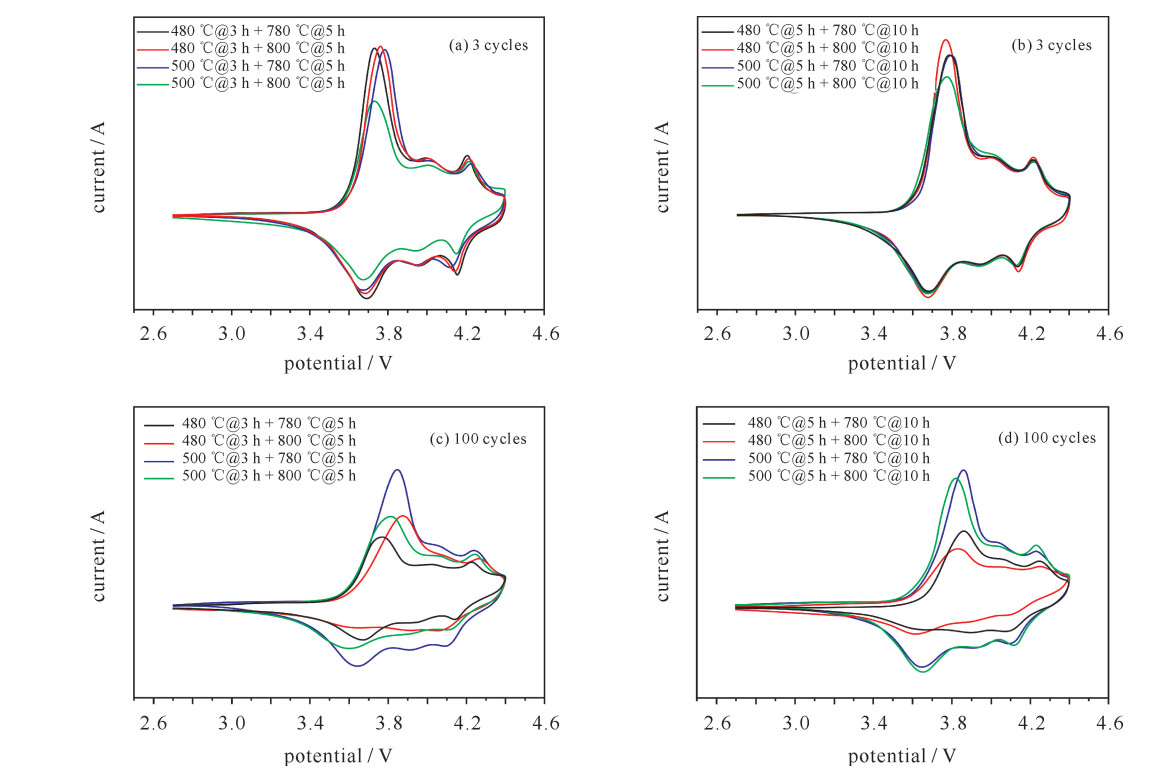
sample oxidation peak potential/V reduction peak potential/V potential(oxidation peak-reduction peak)/V 3 cycles 100 cycles 3 cycles 100 cycles 3 cycles 100 cycles 480 ℃@3 h+780 ℃@5 h 3.733 3.767 3.699 3.669 0.034 0.098 480 ℃@3 h+800 ℃@5 h 3.760 3.872 3.682 3.628 0.078 0.244 500℃@3 h+780 ℃@5 h 3.784 3.846 3.670 3.639 0.114 0.207 500℃@3 h+800 ℃@5 h 3.732 3.817 3.667 3.592 0.065 0.225 480 ℃@5 h+780 ℃@10 h 3.776 3.859 3.681 3.653 0.095 0.206 480 ℃@5 h+800 ℃@10 h 3.769 3.828 3.680 3.626 0.089 0.202 500℃@5 h+780 ℃@10 h 3.794 3.859 3.679 3.644 0.115 0.215 500℃@5 h+800 ℃@10 h 3.780 3.823 3.680 3.652 0.100 0.171 表 5 三元正极材料电化学性能对比表
Table 5. Properties comparison of ternary layered oxide cathode materials
ternary anode material initial charge-discharge specific capacity at 0.1C magnification/(mA·h·g-1) capacity retention ratio after 100 cycles at 1C/% ref. LiNi0.6Co0.2Mn0.2O2 173.0 89.7 [23] LiNi0.5Co0.2Mn0.3O2 167.4 89.7 [24] LiNi1/3Co1/3Mn1/3O2 152.3 85.8 [25] LiNi0.4Co0.2Mn0.4O2 180.7 90.3 [26] LiNi1-x-yCoxAlyO2 172.7 92.1 [27] LiNi0.8Co0.15Mn0.05O2 200.0 94.0 -
[1] 闫金定. 锂离子电池发展现状及其前景分析[J]. 航空学报, 2014, 35(10): 2767-2775. https://www.cnki.com.cn/Article/CJFDTOTAL-HKXB201410008.htmYan Jinding. Current status and development analysis of lithium-ion batteries. Acta Aeronautica et Astronautica Sinica, 2014, 35(10): 2767-2775 https://www.cnki.com.cn/Article/CJFDTOTAL-HKXB201410008.htm [2] Li Yangxing, Wan Chunrong, Wu Yuping, et al. Synthesis and characterization of ultrafine LiCoO2 powders by a spray-drying method[J]. Journal of Power Sources, 2000, 85(2): 294-298. doi: 10.1016/S0378-7753(99)00159-7 [3] Armand M, Tarascon J M. Building better batteries[J]. Nature, 2008, 451: 652-657. doi: 10.1038/451652a [4] 庞春会, 吴川, 吴锋, 等. 锂离子电池纳米正极材料合成方法研究进展[J]. 硅酸盐学报, 2012, 40(2): 247-255. doi: 10.14062/j.issn.0454-5648.2012.02.012Pang Chunhui, Wu Chuan, Wu Feng, et al. Development on synthesis methods for nano-materials of cathode for lithium ion battery. Journal of the Chinese Ceramic Society, 2012, 40(2): 247-255 doi: 10.14062/j.issn.0454-5648.2012.02.012 [5] Patil A, Patil V, Shin D W, et al. Issue and challenges facing rechargeable thin film lithium batteries[J]. Mater Res Bull, 2008, 43: 1913-1942. doi: 10.1016/j.materresbull.2007.08.031 [6] Shukla A K, Kumar T P. Materials for next-generation lithium batteries[J]. Curr Sci, 2008, 94: 314-331. [7] Hassoun J, Kim J H, Lee D J. A contribution to the progress of high energy batteries: A metal-free, lithium-ion, silicon-sulfur battery[J]. Journal of Power Sources, 2012, 202: 308-313. doi: 10.1016/j.jpowsour.2011.11.060 [8] 邹邦坤, 丁楚雄, 陈春华. 锂离子电池三元正极材料的研究进展[J]. 中国科学: 化学, 2014, 44(7): 1104-1115. https://www.cnki.com.cn/Article/CJFDTOTAL-JBXK201407005.htmZou Bangkun, Ding Chuxiong, Chen Chunhua. Research progress in ternary cathode materials Li(Ni, Co, Mn)O2 for lithium ion batteries. Scientia Sinica Chimica, 2014, 44(7): 1104-1115 https://www.cnki.com.cn/Article/CJFDTOTAL-JBXK201407005.htm [9] 黄可龙, 王兆翔, 刘素琴. 锂离子电池原理与关键技术[M]. 北京: 化学工业出版社, 2008: 1-10.Huang Kelong, Wang Zhaoxiang, Liu Suqin. Principle and key technology of lithium ion battery. Beijing: Chemical Industry Press, 2008: 1-10 [10] 马璨, 吕迎春, 李泓. 锂离子电池基础科学问题(Ⅶ)—正极材料[J]. 储能科学与技术, 2014, 3(1): 53-65. doi: 10.3969/j.issn.2095-4239.2014.01.008Ma Can, Lü Yingchun, Li Hong. Fundamental scientific aspects of lithium batteries (Ⅶ)—positive electrode materials. Energy Storage Science and Technology, 2014, 3(1): 53-65 doi: 10.3969/j.issn.2095-4239.2014.01.008 [11] 章福平, 纪勇, 李安东, 等. 锂离子电池正极材料研究的新动向和挑战[J]. 化学通报, 2011, 74(10): 891. https://www.cnki.com.cn/Article/CJFDTOTAL-HXTB201110005.htmZhang Fuping, Ji Yong, Li Andong, et al. Progresses and challenges in studies on cathodic materials of Li-ion battery. Chemistry, 2011, 74(10): 891 https://www.cnki.com.cn/Article/CJFDTOTAL-HXTB201110005.htm [12] Wang Zhao. Studies on Li-excess Mn-based Li1. 2Mn0.54Ni0.13Co0.13O2 as cathode materials for lithium batteries. Beijing: Beijing Institute of Technology, 2015: 1-189 [13] 周燕芳, 钟辉. 锂离子电池正极材料的研究进展[J]. 材料开发与应用, 2003, 18(2): 34-38. doi: 10.3969/j.issn.1003-1545.2003.02.012Zhou Yanfang, Zhong Hui. Research progress in positive electrode materials for lithium ion battery. Development and Application of Materials, 2003, 18(2): 34-38 doi: 10.3969/j.issn.1003-1545.2003.02.012 [14] Muto S S, Tatsumi K S, Kojima Y J, et al. Effect of Mg-doping on the degradation of LiNiO2-based cathode materials by combined spectroscopic methods[J]. Power Sources, 2012, 205: 449-455. doi: 10.1016/j.jpowsour.2012.01.071 [15] Cui Y L, Bao W J, Yuan Z, et al. Comparison of different soft chemical routes synthesis of submicro-LiMn2O4 and their influence on its electrochemical properties[J]. Solid State Electrochemistry, 2012, 16(4): 1551-1559. doi: 10.1007/s10008-011-1558-6 [16] 周文彩, 李金洪, 姜晓谦. 磷酸铁锂制备工艺及研究进展[J]. 硅酸盐通报, 2010, 29(1): 134-146. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201001031.htmZhou Wencai, Li Jinhong, Jiang Xiaoqian. Preparation and research progress of LiFePO4 cathode material. Bulletin of the Chinese Ceramic Society, 2010, 29(1): 134-146 https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201001031.htm [17] 张新龙, 胡国荣, 彭忠东, 等. 锂离子电池正极材料LiFePO4的研究进展[J]. 电池, 2003, 33(4): 252-254. https://www.cnki.com.cn/Article/CJFDTOTAL-DACI200304020.htmZhang Xinlong, Hu Guo-rong, Peng Zhongdong, et al. Development of LiFePO4 as cathode material of Li-ion battery. Battery Bimonthly, 2003, 33(4): 252-254 https://www.cnki.com.cn/Article/CJFDTOTAL-DACI200304020.htm [18] 沙鸥, 赵敏寿, 翟静, 等. 锂离子电池新型正极材料LiFePO4的研究进展[J]. 稀有金属材料与工程, 2009, 38(11): 252-254. https://www.cnki.com.cn/Article/CJFDTOTAL-COSE200911043.htmSha Ou, Zhao Minshou, Zhai Jing, et al. Research progress on LiFePO4—A new type of cathode materials for lithium-ion battery. Rare Metal Materials and Engineering, 2009, 38(11): 252-254 https://www.cnki.com.cn/Article/CJFDTOTAL-COSE200911043.htm [19] 翟静, 赵敏寿, 沙鸥, 等. 锂离子电池正极材料Li3V2(PO4)3的研究进展[J]. 稀有金属材料与工程, 2010, 39(7): 1311-1316. https://www.cnki.com.cn/Article/CJFDTOTAL-COSE201007040.htmZhai Jing, Zhao Minshou, Sha Ou, et al. Research progress in cathode material Li3V2(PO4)3 for Li-ion batteries. Rare Metal Materials and Engineering, 2010, 39(7): 1311-1316 https://www.cnki.com.cn/Article/CJFDTOTAL-COSE201007040.htm [20] Li Y J, Hong L, Wu F. Preparation of Li3V2(PO4)3 cathode material for power Li-ion batteries[J]. Progress in Chemistry, 2012, 24(1): 47-53. [21] 李龙. 锂离子电池镍钴锰三元正极材料的合成与改性研究[D]. 北京: 清华大学, 2012: 1-54.Li Long. Synthesis and modification of LiNixCoyMnzO2 cathode material for lithium battery. Beijing: Tsinghua University, 2012: 1-54 [22] Ruan Zewen. Synthesis and modification of LiNi1-x-yCoxAlyO2 nickel rich cathode materials. Harbin: Harbin Institute of Technology, 2016: 1-81 [23] 艾灵, 毛丽萍, 李世友, 等. 三元正极材料LiNi0.6Co0.2Mn0.2O2制备及改性方法进展[J]. 化工新型材料, 2018, 46(5): 11-15. https://www.cnki.com.cn/Article/CJFDTOTAL-HGXC201805003.htmAi Ling, Mao Liping, Li Shiyou, et al. Progress on the preparation and modification of LiNi0.6Co0.2Mn0.2O2 ternary anode material. New Chemical Materials, 2018, 46(5): 11-15 https://www.cnki.com.cn/Article/CJFDTOTAL-HGXC201805003.htm [24] 李尚津. 锂离子电池三元正极材料LiNi0.5Co0.2Mn0.3O2的制备与改性[D]. 南宁: 广西大学: 2017: 1-47.Li Shangjin. Preparation and modification of LiNi0.5Co0.2Mn0.3O2 of the lithium ion battery. Nanning: Guangxi University, 2017: 1-47 [25] 路乃群, 王存国, 王静强, 等. 锂离子电池三元正极材料LiNi1/3Co1/3Mn1/3O2的制备方法及其改性研究进展[J]. 化工科技, 2018, 26(6): 58-63. https://www.cnki.com.cn/Article/CJFDTOTAL-JKGH201806013.htmLu Naiqun, Wang Cunguo, Wang Jingqiang, et al. Development of the ternary cathode material LiNi1/3Co1/3Mn1/3O2 and its modification for lithium ion batteries. Science & Technology in Chemical Industry, 2018, 26(6): 58-63 https://www.cnki.com.cn/Article/CJFDTOTAL-JKGH201806013.htm [26] Wolff-Goodrich S, Lin F, Markus I M, et al. Tailoring the surface properties of LiNi0.4Mn0.4Co0.2O2 by titanium substitution for improved high voltage cycling performance[J]. Physical Chemistry Chemical Physics, 2015, 17(34): 21778-21781. [27] 王义飞, 武行兵, 王双双, 等. 三元正极材料LiNi1- x- yCoxAlyO的研究进展[J]. 电池, 2017, 47(2): 112-115. https://www.cnki.com.cn/Article/CJFDTOTAL-DACI201702017.htmWang Yifei, Wu Xingbing, Wang Shuangshuang, et al. Research progress in ternary cathode materials LiNi1-x-yCoxAlyO2. Battery Bimonthly, 2017, 47(2): 112-115 https://www.cnki.com.cn/Article/CJFDTOTAL-DACI201702017.htm -





 下载:
下载: