Plasma nitriding of depleted uranium
-
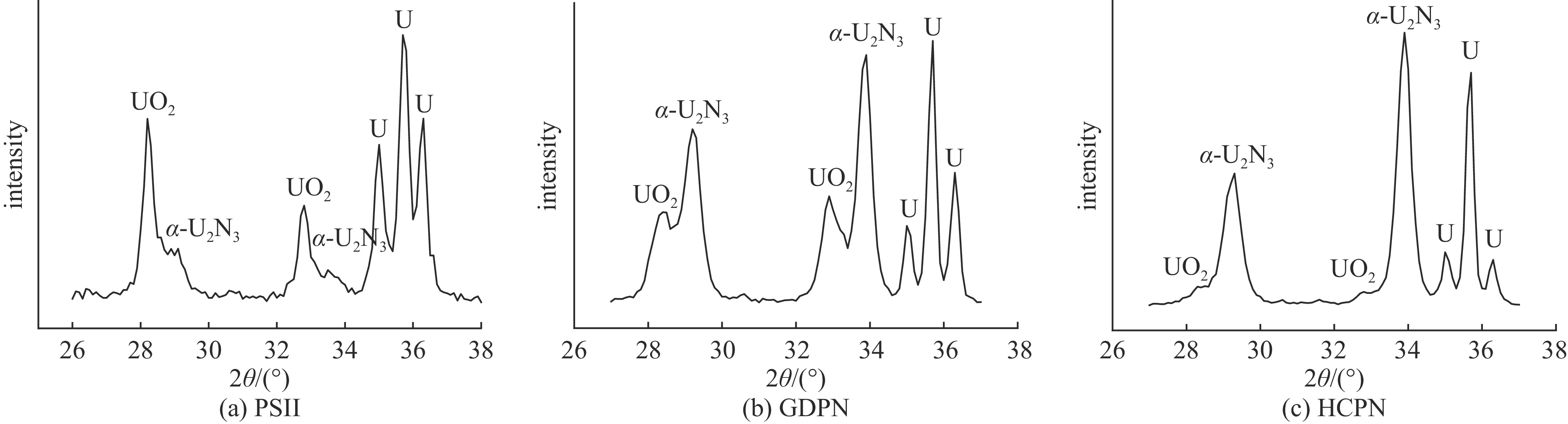
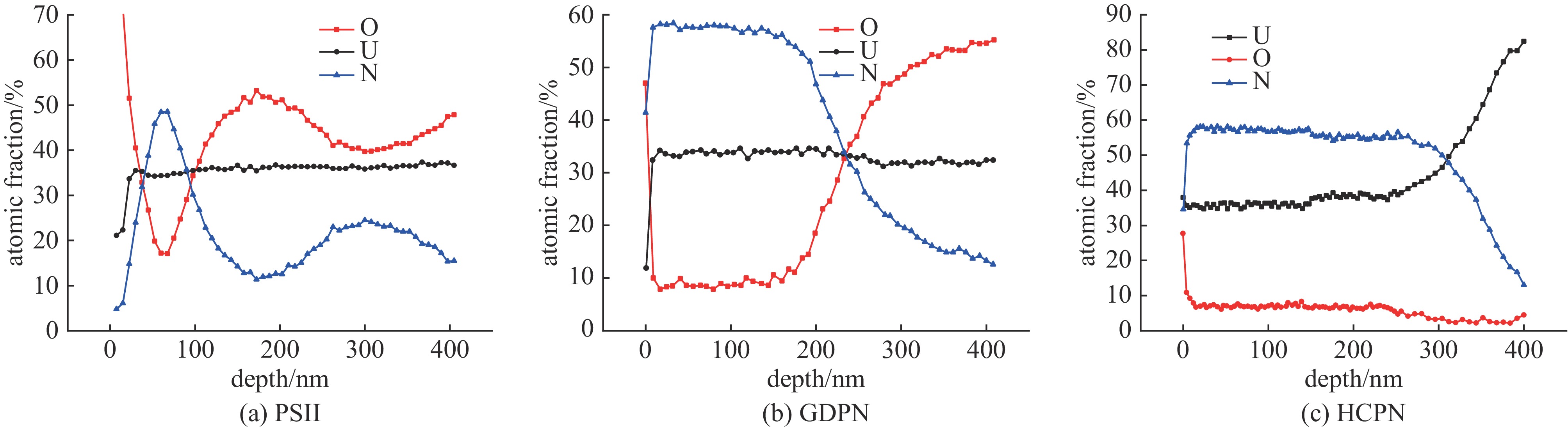
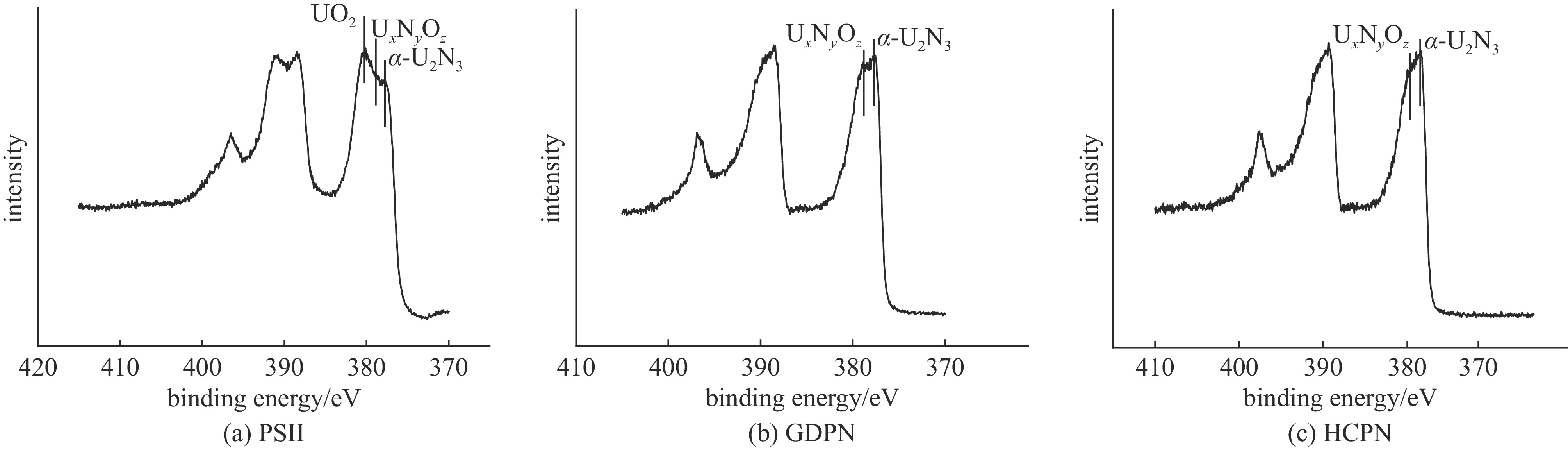
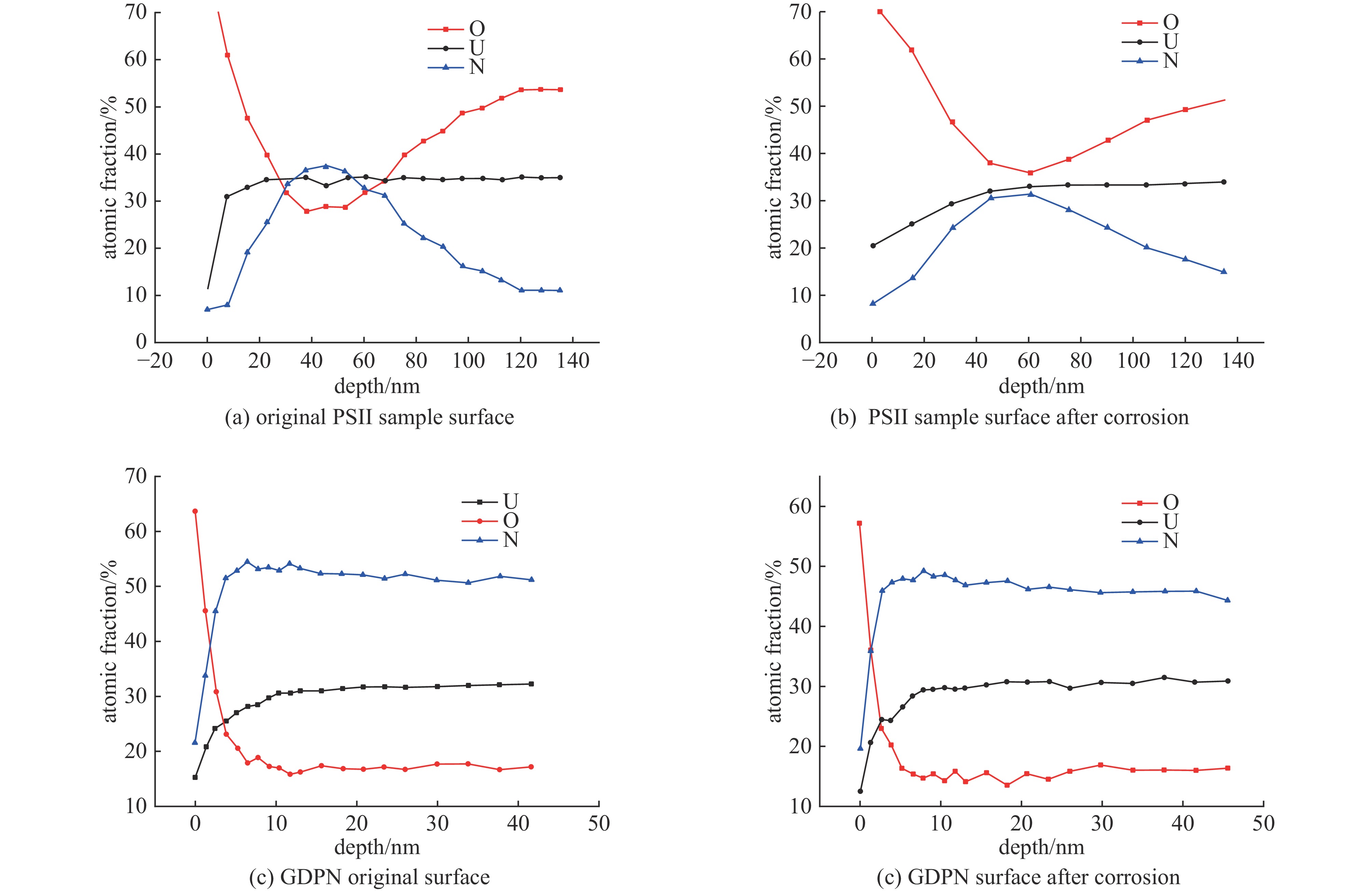
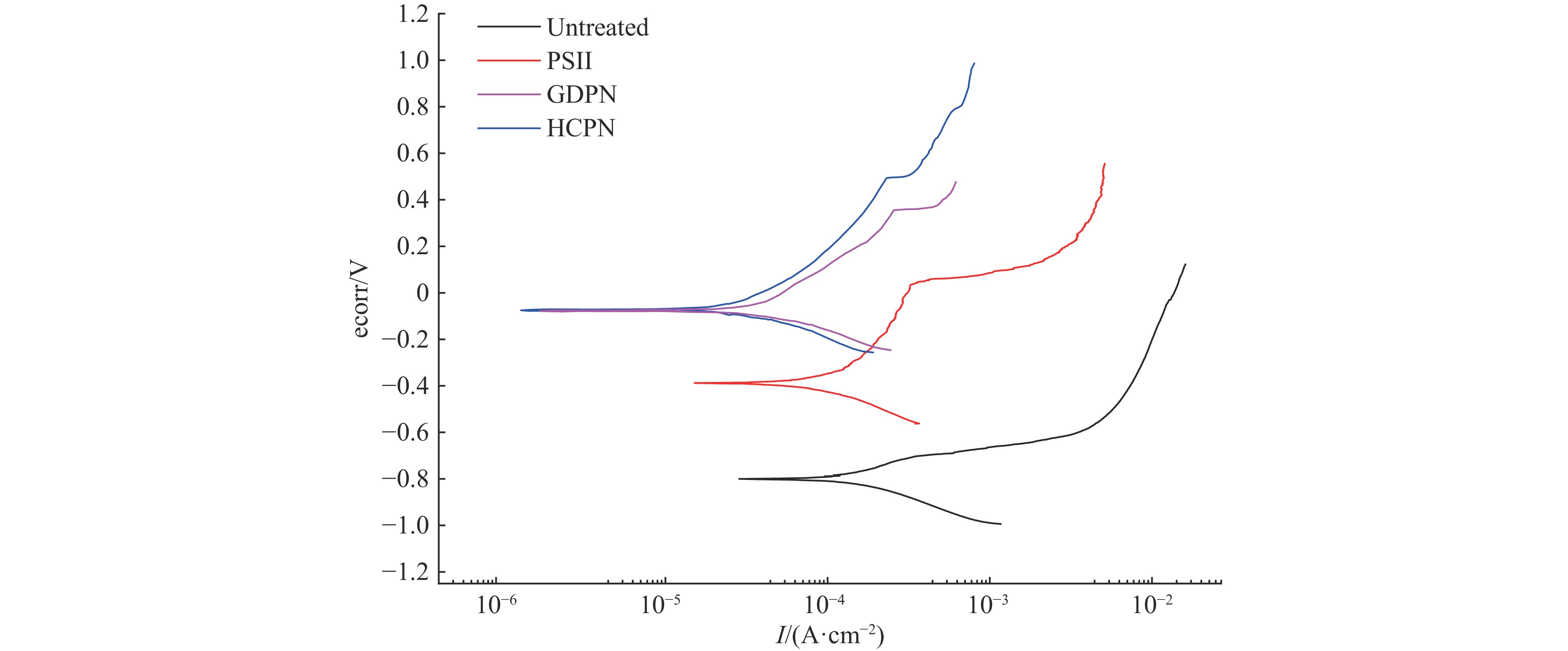
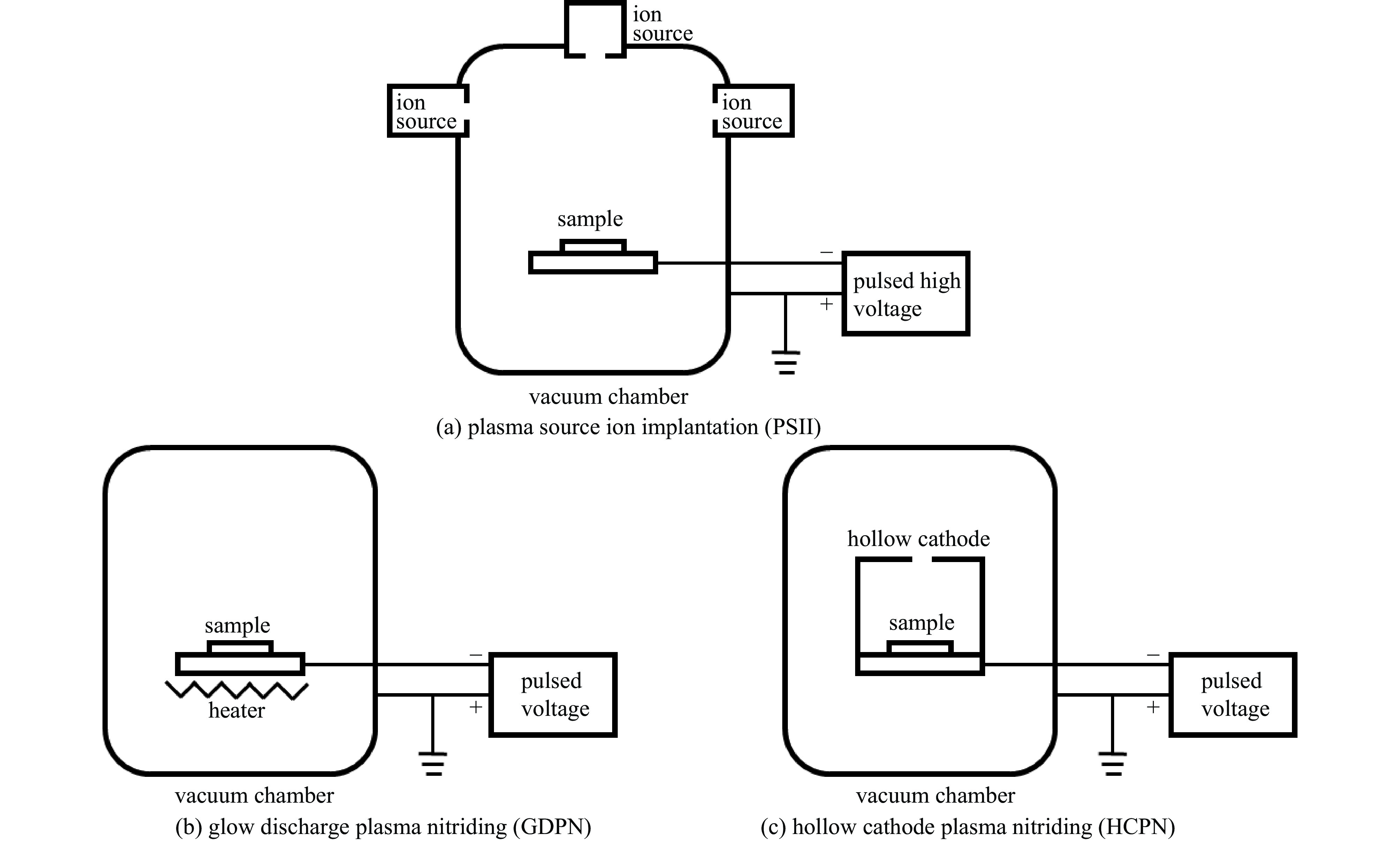
摘要: 为了提高贫化铀表面耐腐蚀性能,利用三种等离子氮化技术(等离子源离子注入Plasma Source Ion Implantation——PSII、辉光放电等离子氮化Glow Discharge Plasma Nitriding——GDPN、空心阴极等离子氮化Hollow Cathode Plasma Nitriding——HCPN)在贫化铀表面制备了氮化层。通过多种材料分析手段对氮化层成分、结构、化学状态进行了分析。三种氮化层中的氮化物都以α-U2N3为主,由于金属铀与氧的亲和力较强,三种等离子氮化过程都不同程度地引入了氧杂质。PSII可以突破热力学平衡,将部分氧化物转化为氮化物。GDPN和HCPN则通过表面反应和热扩散形成氮化物层。HCPN技术对控制氧杂质有一定优势,可以显著降低氮化层中氧杂质含量。湿热腐蚀和电化学测试表明,等离子氮化可以明显提升贫化铀的抗腐蚀性能,HCPN和GDPN的提升程度要优于PSII,而HCPN技术最优。本文的研究结果可为活性金属的等离子氮化处理提供参考。Abstract: To enhance the corrosion resistance of depleted uranium surfaces, a comparative analysis was conducted to evaluate the efficacy of three plasma nitriding technologies: Plasma Source Ion Implantation (PSII), Glow Discharge Plasma Nitriding (GDPN), and Hollow Cathode Plasma Nitriding (GDPN). The composition, structure and chemical state of the nitrided layers were analysed using a range of material analysis methods. The nitrides present in the three nitrided layers are predominantly α-U2N3. Due to the strong affinity between uranium metal and oxygen, all three plasma nitriding processes have introduced oxygen impurities to varying degrees. PSII is capable of breaking through the thermodynamic equilibrium and converting some of the oxides into nitrides, while GDPN and HCPN can form nitrides through surface reactions and thermal diffusion. HCPN technology has certain advantages in controlling the oxygen impurities, and can significantly reduce the oxygen impurities in the nitrided layer. The results of the wet heat corrosion and electrochemical tests demonstrate that plasma nitriding can markedly enhance the corrosion resistance of depleted uranium. The degree of improvement achieved by HCPN and GDPN is superior to that of PSII, with HCPN technology exhibiting the most favourable outcome. The findings of this study could provide a reference for plasma nitriding treatment of reactive metals..
-
Key words:
- depleted uranium /
- plasma nitriding /
- surface treatment /
- corrosion resistance
-
表 1 实验材料杂质
Table 1. Impurities of experimental materials
experimental
materialimpurity content/10−6 carbon nitrogen oxygen H2O others uranium 200 45 <150 nitrogen <3 <3 <5 <1 表 2 主要工艺参数
Table 2. Process parameters
process base
presure/Pavoltage/
kVfrequence/
kHzpulse
width/μscurrent density/
(mA·cm−2)process
time/hvacuum
temperature/℃PSII 8×10−4 50 0.4 40 ~0.1 2 220 GDPN 3×10−4 0.9 60 1.5 ~1 2 350 HCPN 3×10−4 0.6 60 0.5 ~20 2 130 -
[1] Colmenares C A. The oxidation of thorium, uranium, and plutonium[J]. Progress in Solid State Chemistry, 1975, 9: 139-239. doi: 10.1016/0079-6786(75)90016-3 [2] Mattox D M, Bland R D. Aluminum coating of uranium reactor parts for corrosion protection[J]. Journal of Nuclear Materials, 1967, 21(3): 349-352. doi: 10.1016/0022-3115(67)90189-4 [3] Chang F, Levy M, Jackman B, et al. Assessment of corrosion-resistant coatings for a depleted uranium-0.75 titanium alloy[J]. Surface and Coatings Technology, 1991, 48(1): 31-39. doi: 10.1016/0257-8972(91)90126-H [4] Chang F C, Levy M, Huie R, et al. Adhesion and corrosion behavior of Al-Zn and TiN/Ti/TiN coatings on a DU-0.75wt. % Ti alloy[J]. Surface and Coatings Technology, 1991, 49(1/3): 87-96. [5] Musket R G. Applications of ion implantation for modifying the interactions between metals and hydrogen gas[J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 1989, 40/41: 591-594. [6] Crusset D, Bernard F, Sciora E, et al. Modification of the hydriding kinetics of U-0.2wt. %V alloy using ion implantations[J]. Journal of Alloys and Compounds, 1994, 204(1/2): 71-77. [7] Arkush R, Mintz M H, Shamir N. Passivation of uranium towards air corrosion by N2+ and C+ ion implantation[J]. Journal of Nuclear Materials, 2000, 281(2/3): 182-190. [8] Arkush R, Brill M, Zalkind S, et al. The effect of N2+ and C+ implantation on uranium hydride nucleation and growth kinetics[J]. Journal of Alloys and Compounds, 2002, 330/332: 472-475. doi: 10.1016/S0925-8388(01)01537-7 [9] Arkush R, Mintz M H, Kimmel G, et al. Long-term amorphisation of C+ and N2+ implanted layers on a uranium surface[J]. Journal of Alloys and Compounds, 2002, 340(1/2): 122-126. [10] Raveh A, Arkush R, Zalkind S, et al. Passivation of uranium metal by radio-frequency plasma nitriding against gas phase (H2, H2O) corrosion[J]. Surface and Coatings Technology, 1996, 82(1/2): 38-41. [11] Yeamans C B, Silva G W C, Cerefice G S, et al. Oxidative ammonolysis of uranium(IV) fluorides to uranium(VI) nitride[J]. Journal of Nuclear Materials, 2008, 374(1/2): 75-78. [12] Silva G W C, Yeamans C B, Ma Longzhou, et al. Microscopic characterization of uranium nitrides synthesized by oxidative ammonolysis of uranium tetrafluoride[J]. Chemistry of Materials, 2008, 20(9): 3076-3084. doi: 10.1021/cm7033646 [13] Black L, Miserque F, Gouder T, et al. Preparation and photoelectron spectroscopy study of UN x thin films[J]. Journal of Alloys and Compounds, 2001, 315(1/2): 36-41. [14] Mändl S, Gerlach J W, Assmann W, et al. Phase formation and diffusion after nitrogen PIII in molybdenum[J]. Surface and Coatings Technology, 2003, 174/175: 1238-1242. doi: 10.1016/S0257-8972(03)00457-2 [15] Möller W, Parascandola S, Kruse O, et al. Plasma-immersion ion implantation for diffusive treatment[J]. Surface and Coatings Technology, 1999, 116/199: 1-10. [16] Liu Kezhao, Wang Xiaofang, Liu Jing, et al. Nitride layers on uranium surfaces[J]. Progress in Surface Science, 2018, 93(3): 47-84. doi: 10.1016/j.progsurf.2018.08.002 [17] Long Zhong, Luo Lizhu, Lu Yong, et al. Study on the electronic structure of α-U2N3 by XPS and first principles[J]. Journal of Alloys and Compounds, 2016, 664: 745-749. doi: 10.1016/j.jallcom.2016.01.013 -




 下载:
下载: