Design and validation of a proton beam line based on a rapid-cycling synchrotron for Flash radiation
-
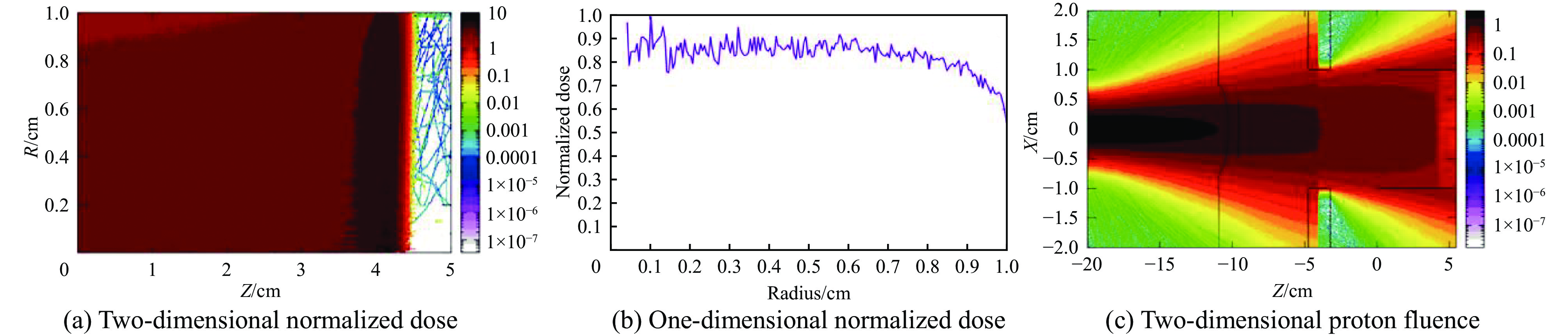
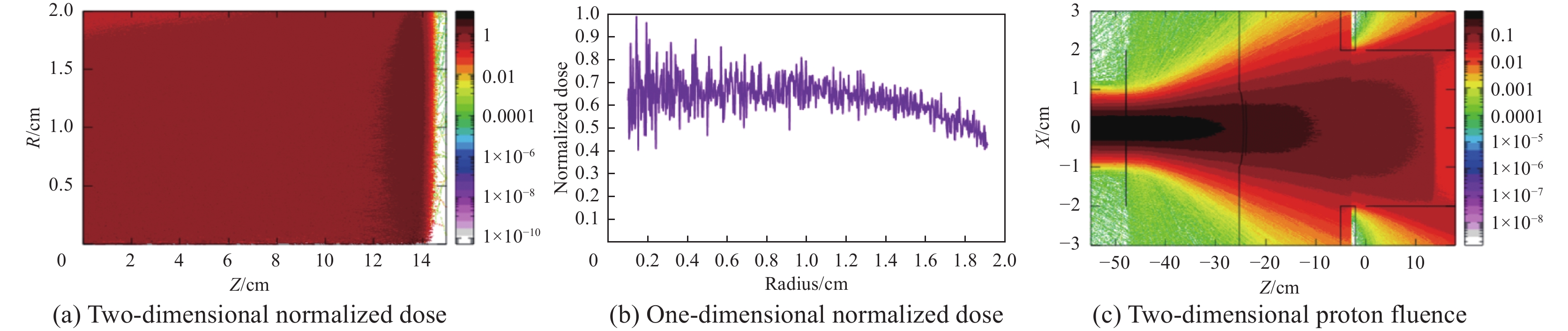
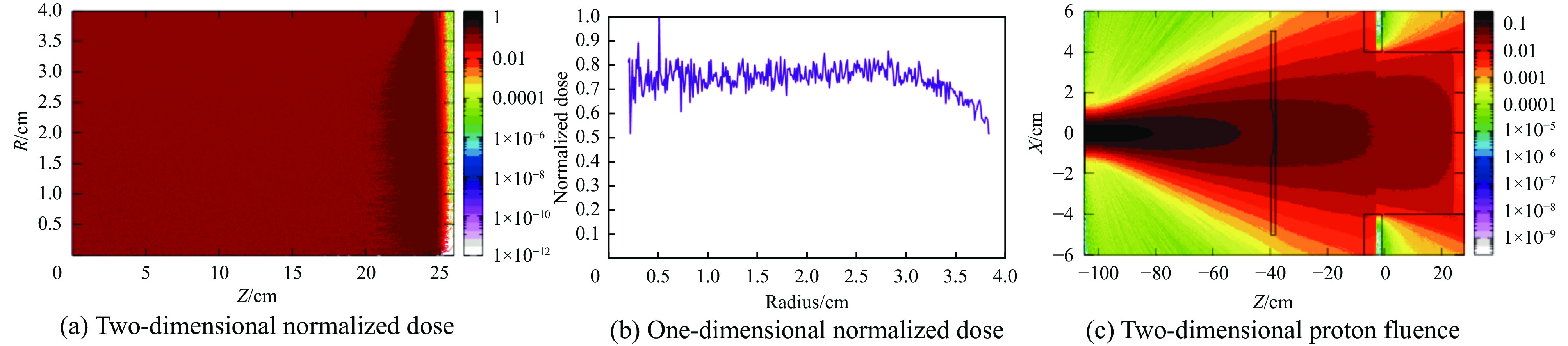
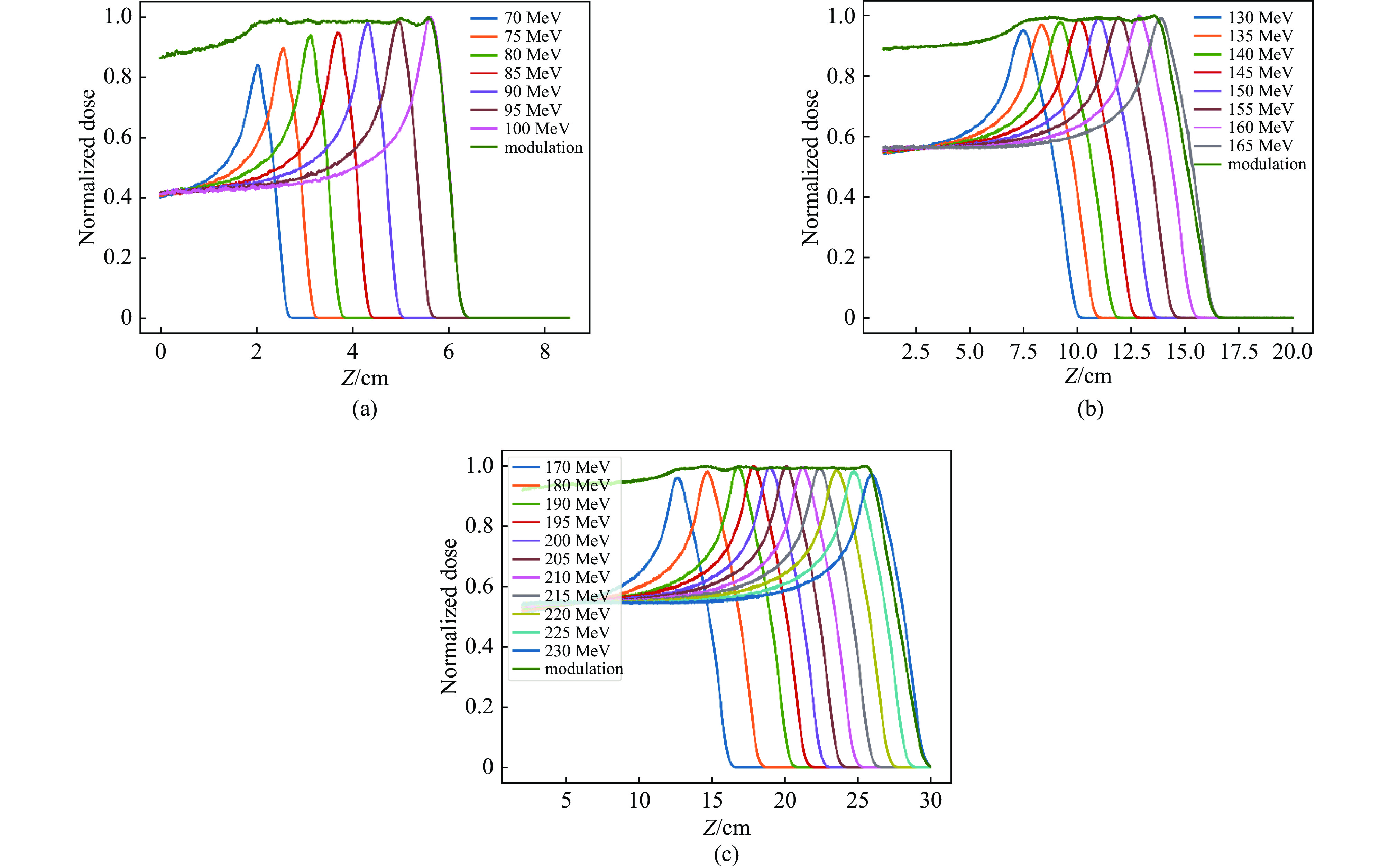
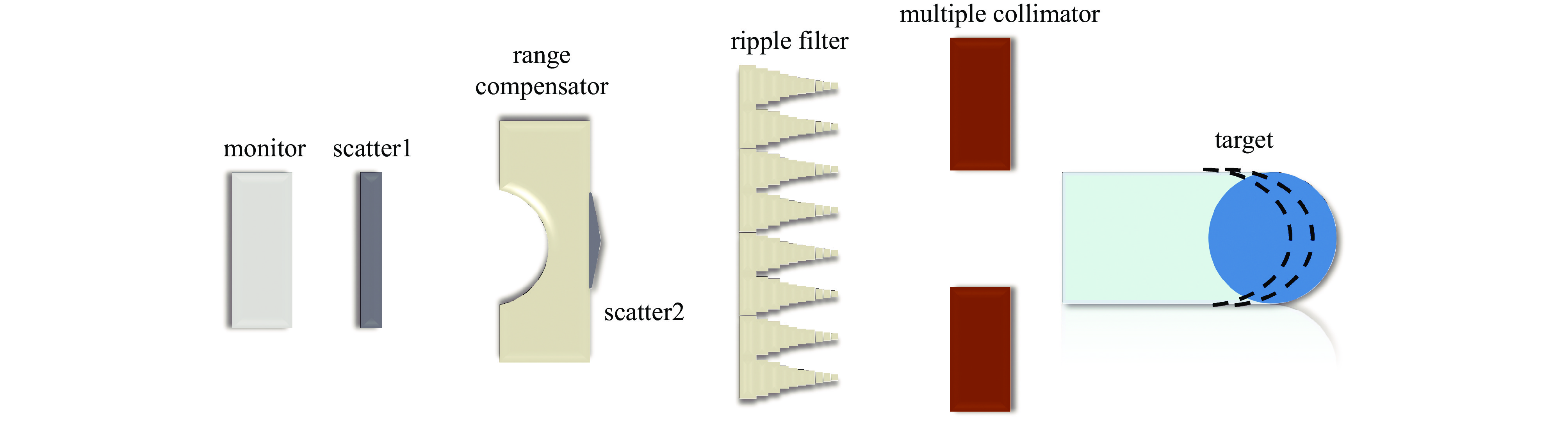
摘要: 为了实现超高剂量率的质子Flash照射,基于快循环同步加速器建立了一个束流配送系统。快循环同步加速器能够在数百ns内快速引出质子束,通过改变不同的引出时间引出不同能量的束流,从而实现能量的快速切换。基于这个特性,考虑与层叠加照射方式相结合,束流的瞬时剂量率可以达到107 Gy/s。靶区在纵向上分成单独的层,每一层需要不同的能量。由于能量层切换的时间非常短,射程调制轮无法满足需求,选用纹波过滤器进行射程调制。使用蒙特卡罗软件FLUKA模拟了整个装置,包括了散射片,射程补偿器,纹波过滤器和准直器,最大化提高进入靶区的质子通量。在低、中、高三个能量区域,根据原始布拉格峰曲线设计了三种尺寸的纹波过滤器,将尖峰区域扩展成高斯分布,分别提供了2、6、13 cm宽度的三个扩展布拉格峰区域,有效减少了能量层数量,缩短了整体照射时间。将快循环同步加速器与层叠加的照射方式相结合,可以获得超高瞬时剂量率的照射野,为实现Flash照射提供了一种新的方法。Abstract: We design a proton beamline based on a rapid-cycling synchrotron for Flash radiation with ultra-high dose rate. Because proton beams can be extracted within hundreds of nanoseconds in the rapid-cycling synchrotron, its energy may be altered from one cycle to the next with different extraction time. The intended beamline system can realize layer stacking irradiation at an instantaneous dose rate of 107 Gy/s. Each of longitudinal layer requires a different beam intensity. The target is divided longitudinally into different layers, each of which needs a different beam energy, in order to produce a uniform irradiation field. The system, including a double scatter system, a range compensator, a ripple filter, and a multi-leaf collimator to maximize proton fluence into the target, is simulated using the Monte Carlo software FLUKA. Three different kinds of ripple filters are built in the low, medium, and high energy zones based on the original Bragg peaks in order to decrease the number of energy layers and shorten the total irradiation duration. These filters transform the spike region into a Gaussian distribution with flat expansion areas of 2 cm, 6 cm, and 20 cm, respectively. Combining the rapid-cycling synchrotron with the layer stacking irradiation provides a novel method for realizing Flash proton irradiation, which delivers an ultra-high dose rate to the target.
-
Key words:
- proton therapy /
- Flash effect /
- beam delivery system /
- layer stacking /
- rapid-cycling synchrotron
-
表 1 三个不同能量的质子束经过有无固体水的水箱测量数据和FLUKA模拟结果对比
Table 1. Comparison of observed data using the water phantom and FLUKA simulation results about weather passing through solid water under three different energy levels
Energy/MeV With 5 cm solid
water or noBragg peak/cm R80_1/cm R80_2/cm ΔR80/cm 109 no Measurement 8.70419 8.54409 8.80425 0.26016 Simulation 8.70218 8.54214 8.80220 0.26007 with Measurement 3.64103 3.46091 3.74109 0.28018 Simulation 3.65091 3.49087 3.75094 0.26007 152 no Measurement 15.79053 15.54036 16.00067 0.46031 Simulation 15.80395 15.53388 15.98400 0.45011 with Measurement 10.75829 10.47688 10.92915 0.45226 Simulation 10.77269 10.48262 10.93273 0.45011 235 no Measurement 33.93419 33.37339 34.27468 0.90129 Simulation 33.89847 33.34834 34.25856 0.91023 with Measurement 28.85285 28.31231 29.19319 0.88088 Simulation 28.87722 28.31708 29.21730 0.90023 表 2 低能区域不同能量质子束的权重
Table 2. Weights of proton beams with different energies in low energy region
Energy/MeV Weight Flatness/% 70 0.042 1.68 75 0.048 2.43 80 0.062 3.12 85 0.082 3.49 90 0.114 3.65 95 0.182 3.72 100 0.470 4.90 表 3 中能区域不同能量质子束的权重
Table 3. Weights of proton beams with different energies in medium energy region
Energy/MeV Weight Flatness/% 130 0.064 5.88 135 0.024 3.57 140 0.036 3.82 145 0.071 2.25 150 0.072 1.12 155 0.104 3.91 160 0.151 4.25 165 0.478 4.88 表 4 高能区域不同能量质子束的权重
Table 4. Weights of proton beams with different energies in high energy region
Energy/MeV Weight Flatness/% 170 0.037 5.69 180 0.030 5.41 190 0.047 4.52 195 0.023 3.48 200 0.025 3.10 205 0.058 2.70 210 0.056 2.85 215 0.052 3.41 220 0.121 4.31 225 0.125 4.82 230 0.426 5.38 -
[1] Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice[J]. Science Translational Medicine, 2014, 6: 245ra93. [2] Allen B D, Alaghband Y, Kramár E A, et al. Elucidating the neurological mechanism of the Flash effect in juvenile mice exposed to hypofractionated radiotherapy[J]. Neuro-Oncology, 2023, 25(5): 927-939. doi: 10.1093/neuonc/noac248 [3] Ashraf M R, Melemenidis S, Liu K, et al. Multi-Institutional audit of FLASH and conventional dosimetry with a 3D printed anatomically realistic mouse phantom[J]. International Journal of Radiation Oncology · Biology · Physics, 2024, 120(1): 287-300. [4] Zhang Qixian, Gerweck L E, Cascio E, et al. Proton FLASH effects on mouse skin at different oxygen tensions[J]. Physics in Medicine & Biology, 2023, 68: 055010. [5] Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s[J]. Radiotherapy and Oncology, 2017, 124(3): 365-369. doi: 10.1016/j.radonc.2017.05.003 [6] Montay-Gruel P, Acharya M M, Petersson K, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(22): 10943-10951. [7] Röch T F, SZABÓZ, Haffa D, et al. A feasibility study of zebrafish embryo irradiation with laser-accelerated protons[J]. Review of Scientific Instruments, 2020, 91: 063303. doi: 10.1063/5.0008512 [8] Beyreuther E, Brand M, Hans S, et al. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation[J]. Radiotherapy and Oncology, 2019, 139: 46-50. doi: 10.1016/j.radonc.2019.06.024 [9] Vozenin M C, De Fornel P, Petersson K, et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients[J]. Clinical Cancer Research, 2019, 25(1): 35-42. doi: 10.1158/1078-0432.CCR-17-3375 [10] Bourhis J, Sozzi W J, Jorge P G, et al. Treatment of a first patient with FLASH-radiotherapy[J]. Radiotherapy and Oncology, 2019, 139: 18-22. doi: 10.1016/j.radonc.2019.06.019 [11] Mascia A E, Daugherty E C, Zhang Yongbin, et al. Proton FLASH radiotherapy for the treatment of symptomatic bone metastases: the FAST-01 nonrandomized trial[J]. JAMA Oncology, 2023, 9(1): 62-69. doi: 10.1001/jamaoncol.2022.5843 [12] Daugherty E C, Zhang Y, Xiao Z, et al. FLASH radiotherapy for the treatment of symptomatic bone metastases in the thorax (FAST-02): protocol for a prospective study of a novel radiotherapy approach[J]. Radiation Oncology, 2024, 19: 34. doi: 10.1186/s13014-024-02419-4 [13] Hughes J R, Parsons J L. FLASH radiotherapy: current knowledge and future insights using proton-beam therapy[J]. International Journal of Molecular Sciences, 2020, 21: 6492. doi: 10.3390/ijms21186492 [14] Borghini A, Labate L, Piccinini S, et al. FLASH radiotherapy: expectations, challenges, and current knowledge[J]. International Journal of Molecular Sciences, 2024, 25: 2546. doi: 10.3390/ijms25052546 [15] Vozenin M C, Bourhis J, Durante M. Towards clinical translation of FLASH radiotherapy[J]. Nature Reviews Clinical Oncology, 2022, 19(12): 791-803. doi: 10.1038/s41571-022-00697-z [16] Jolly S, Owen H, Schippers M, et al. Technical challenges for FLASH proton therapy[J]. Physica Medica, 2020, 78: 71-82. doi: 10.1016/j.ejmp.2020.08.005 [17] Manti L, Perozziello F M, Borghesi M, et al. The radiobiology of laser-driven particle beams: focus on sub-lethal responses of normal human cells[J]. Journal of Instrumentation, 2017, 12: C03084. doi: 10.1088/1748-0221/12/03/C03084 [18] Bayart E, Flacco A, Delmas O, et al. Fast dose fractionation using ultra-short laser accelerated proton pulses can increase cancer cell mortality, which relies on functional PARP1 protein[J]. Scientific Reports, 2019, 9: 10132. doi: 10.1038/s41598-019-46512-1 [19] Bin Jianhui, Obst-Huebl L, Mao Jianhua, et al. A new platform for ultra-high dose rate radiobiological research using the BELLA PW laser proton beamline[J]. Scientific Reports, 2022, 12: 1484. doi: 10.1038/s41598-022-05181-3 [20] Shi Ying, Zhang Manzhou, Ouyang Lianhua, et al. Design of a rapid-cycling synchrotron for Flash proton therapy[J]. Nuclear Science and Techniques, 2023, 34: 145. doi: 10.1007/s41365-023-01283-3 [21] Mori S, Kanematsu N, Asakura H, et al. Four-Dimensional lung treatment planning in layer-stacking carbon ion beam treatment: comparison of layer-stacking and conventional ungated/gated irradiations[J]. International Journal of Radiation Oncology · Biology · Physics, 2011, 80(2): 597-607. [22] Kubo N, Kubota Y, Oike T, et al. Skin dose reduction by layer-stacking irradiation in carbon ion radiotherapy for parotid tumors[J]. Frontiers in Oncology, 2020, 10: 1396. doi: 10.3389/fonc.2020.01396 [23] Grusell E, Montelius A, Brahme A, et al. A general solution to charged particle beam flattening using an optimized dual-scattering-foil technique, with application to proton therapy beams[J]. Physics in Medicine & Biology, 1994, 39(12): 2201-2216. [24] Wroe A J, Schulte R W, Barnes S, et al. Proton beam scattering system optimization for clinical and research applications[J]. Medical Physics, 2013, 40: 041702. doi: 10.1118/1.4793262 [25] Gottschalk B. On the scattering power of radiotherapy protons[J]. Medical Physics, 2010, 37(1): 352-367. doi: 10.1118/1.3264177 [26] Kainz K K, Antolak J A, Almond P R, et al. Dual scattering foil design for poly-energetic electron beams[J]. Physics in Medicine & Biology, 2005, 50(5): 755-767. [27] Weber U, Kraft G. Design and construction of a ripple filter for a smoothed depth dose distribution in conformal particle therapy[J]. Physics in Medicine & Biology, 1999, 44(11): 2765-2775. [28] Akagi T, Higashi A, Tsugami H, et al. Ridge filter design for proton therapy at Hyogo Ion Beam Medical Center[J]. Physics in Medicine & Biology, 2003, 48(22): N301-N312. [29] Kang M, Pang D. Commissioning and beam characterization of the first gantry-mounted accelerator pencil beam scanning proton system[J]. Medical Physics, 2020, 47(8): 3496-3510. doi: 10.1002/mp.13972 [30] Smith B R, Hyer D E, Hill P M, et al. Secondary neutron dose from a dynamic collimation system during intracranial pencil beam scanning proton therapy: a Monte Carlo investigation[J]. International Journal of Radiation Oncology · Biology · Physics, 2019, 103(1): 241-250. [31] Grewal H S, Ahmad S, Jin H. Performance evaluation of adaptive aperture’s static and dynamic collimation in a compact pencil beam scanning proton therapy system: a dosimetric comparison study for multiple disease sites[J]. Medical Dosimetry, 2021, 46(2): 179-187. doi: 10.1016/j.meddos.2020.11.001 [32] Grewal H S, Ahmad S, Jin H. Characterization of penumbra sharpening and scattering by adaptive aperture for a compact pencil beam scanning proton therapy system[J]. Medical Physics, 2021, 48(4): 1508-1519. doi: 10.1002/mp.14771 -




 下载:
下载: